Boron is used in, e.g., glass, ceramics, medicinal chemistry, or fertilizers. Boron has some properties that are similar to carbon due to the elements’ proximity within the periodic table. Analogous to graphene, boron also has two-dimensional forms, i.e., borophenes, which show interesting properties. Creating one-dimensional structures based on graphene, such as graphene nanoribbons, can be an efficient strategy for introducing new electronic properties. One-dimensional variants of borophenes have also been predicted, but boron allotropes with widths less than 2 nm had not been synthesized so far.
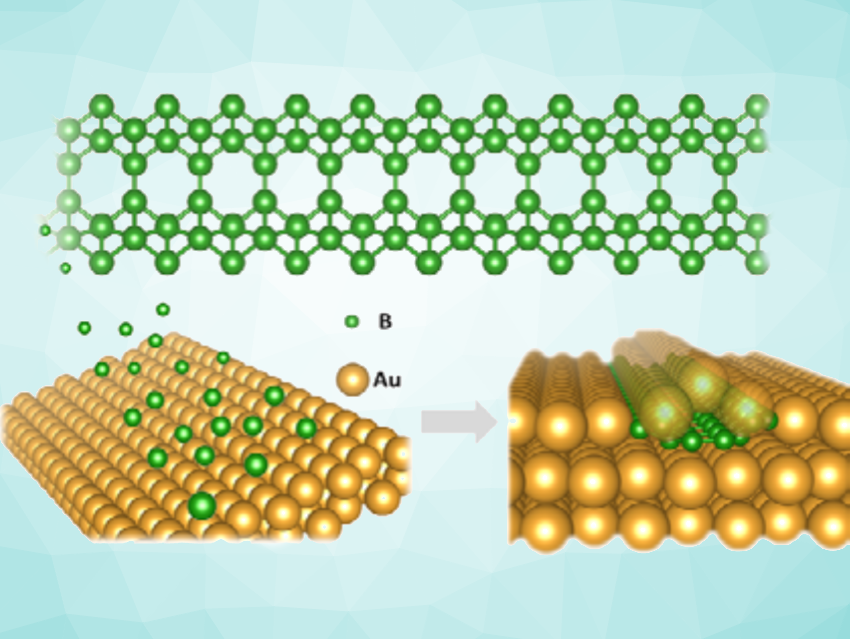
Miao Yu, Zhibo Ma, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Yang-Gang Wang, Xueming Yang, Southern University of Science and Technology, Shenzhen, China, and colleagues have synthesized a new boron structure, super-narrow borophene nanoribbons (BNR). The team evaporated B atoms at ca. 2200 K onto an Au(111) surface held at room temperature. Using scanning tunneling microscopy (STM) imaging, they observed the formation of 1D ribbons with widths of about 1 nm and lengths up to 200 Å.
To understand the structure and formation of the nanoribbons, the researchers used density functional theory (DFT) calculations and ab initio molecular dynamics (AIMD) simulations. They propose that the boron atoms can penetrate the outer layer of the Au(111) surface and form nanoribbons composed of pairs of zigzag (2,2) boron rows (pictured in green).
- Formation of Supernarrow Borophene Nanoribbons,
Haochen Wang, Pengcheng Ding, Guang-Jie Xia, Xiangyun Zhao, Wenlong E, Miao Yu, Zhibo Ma, Yang-Gang Wang, Lai-Sheng Wang, Jun Li, Xueming Yang,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202406535