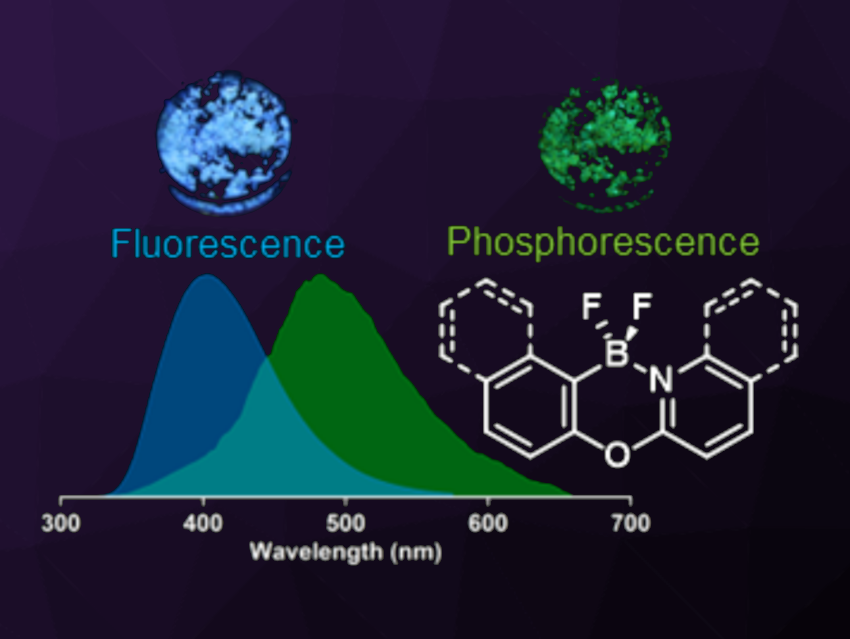
Light-emissive organic materials are most often fluorescent. Realizing and understanding examples of organic compounds that exhibit phosphorescence under ambient conditions is interesting. Organic room temperature phosphorescence (ORTP) can provide the long-lived excited states and wavelength-shifted emission maxima that are characteristic of phosphorescence without the use of transition metals. Most known ORTP materials rely on well-known structural motifs that include aryl carbonyls, sulfones, and heavy main group elements.
Joe B. Gilroy, The University of Western Ontario, London, Canada, and colleagues have developed a series of heavy atom-free BN-substituted xanthene derivatives (general structure pictured) that show ORTP. The team started from corresponding aryl ether precursors, i.e., 2-phenoxypyridine, 2-phenoxyquinoline, and 2-naphthoxyquinoline, and introduced a BBr2 unit via a reaction with BBr3 and i-Pr2NEt. Treatment with Selectfluor then gave the desired derivatives with a BF2 unit (pictured) in yields of 67–78 %.
The products were analyzed using X-ray diffraction, and all three have a conjugated, planar ligand backbone. Photoluminescence studies showed that the compounds exhibit fluorescence in combination with ORTP in the solid state. The researchers propose that close intermolecular interactions in the solid-state structure, e.g., C–H···F interactions inhibit quenching by thermal motion. Overall, the work provides heavy-atom-free organic phosphors that work at room temperature and in an oxygen-containing atmosphere.
- Organic Room Temperature Phosphorescence from BN‐Substituted Xanthene Derivatives,
Alex E. R. Watson, Si Yuan Tao, Alex Siemiarczuk, Paul D. Boyle, Paul J. Ragogna, Joe B. Gilroy,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202414534