Laypeople and experts all agree on nature’s biggest stinker: it is Mephitis mephitis, the striped stunk. It is, therefore, not surprising that few scientists have been bold enough to approach this animal. Friedrich Wöhler described the fate of everyone who entered this field of study: you stink of skunk for days.
The secretion is a highly complex natural substance and a masterpiece of sulfur chemistry. The road to its identification was not an easy one, as we have previously seen in the first part of this article.
5 1897: The Fourth Attempt
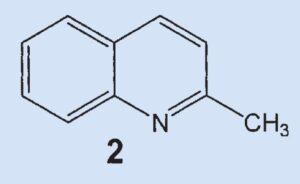 One year later, in 1897 [6], Aldrich and Jones again picked up their study of skunk secretion and identified the higher-boiling compound, 2-methylquinoline (2). This compound is probably the same as the one identified as a nitrogen base by Swarts and Löw (see Part 1). In this case, however, it was possible to determine a definitive structure through comparison with a known, authentic sample.
One year later, in 1897 [6], Aldrich and Jones again picked up their study of skunk secretion and identified the higher-boiling compound, 2-methylquinoline (2). This compound is probably the same as the one identified as a nitrogen base by Swarts and Löw (see Part 1). In this case, however, it was possible to determine a definitive structure through comparison with a known, authentic sample.
In addition, the scientists isolated a second basic component that was difficult to distill over through steam distillation and contains sulfur as well as nitrogen. No further details were given.
Results: the secretion contains 2-methylquinoline (2) and a second sulfur- and nitrogen-containing compound of unknown structure.
6 1945: The Fifth Attempt
Wöhler had previously described the musk-like odor of the urine produced by visiting scientist T. Swarts as he worked with the secretion (see Part 1). The most odorously distinct component of musk, a secretion of the musk deer, is muscone, a fifteen-membered cyclic ketone (3-methylcyclopentadecanone) whose structure was determined by Ruzicka in 1926 [7]. Because of its animal scent, musk remains an irreplaceable and valuable component of top-quality perfumes.
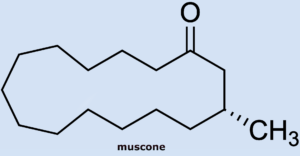 American Philip Stevens [8] took note of Wöhler’s observation and hoped to find larger cyclic ketones in skunk secretion. Unfortunately, he did not find any ketones in any of the fractions.
American Philip Stevens [8] took note of Wöhler’s observation and hoped to find larger cyclic ketones in skunk secretion. Unfortunately, he did not find any ketones in any of the fractions.
An unexpected result of his experiments was that Stevens isolated 4.5 g of a new sulfur-containing compound identified as bis(2-butenyl)sulfide (3).
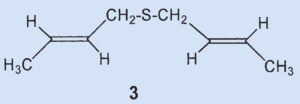
Results: the secretion contains bis(2-butenyl)sulfide (3) but no cyclic ketones analogous to muscone.
All the double bonds described in this article are in the E configuration, which corresponds to a trans arrangement for 1,2-disubstituted alkenes.
7 1975: The Sixth Attempt
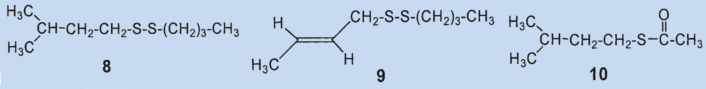
In 1975, Kenneth Andersen and David Bernstein applied gas chromatography as a separation technique to skunk secretion for the first time. This revealed that instead of 1-butanethiol, the primary components of the secretion were 2-butene-1-thiol (4) and 3-methyl-1-butanethiol (5), which together comprised about 66 % of the total secretion [5,9]. The third biggest component was identified as (2-butenyl)methyldisulfide (6), which makes up about 7 % of the mixture.
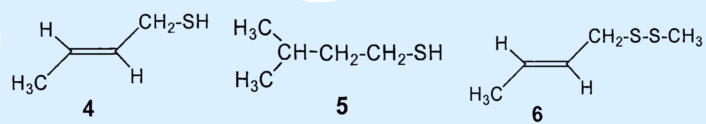
Results: the secretion contains no 1-butanethiol (1); the primary components are 2-butene-1-thiol (4) and 2-methyl-1-butanethiol (5). The third biggest component was identified as (2-butenyl)mehtyldisulfide (6).
8 1982: The Seventh Attempt
In a second study, Andersen and his co-workers employed a new method to identify the fractions they had separated by steam distillation: direct coupling of gas chromatography and mass spectrometry [1]. This allowed them to detect a total of 160 components, 150 of which contain sulfur. The two main components, 2-butene-1-thiol (4) and 3-methyl-1-butanethiol (5) were both confirmed.
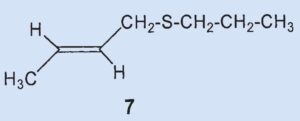 The (2-butenyl)-methyldisulfide (6) that the researchers had previously found could no longer be detected and the third most prevalent component was now (2-butenyl)propylsulfide (7), at 7 %.
The (2-butenyl)-methyldisulfide (6) that the researchers had previously found could no longer be detected and the third most prevalent component was now (2-butenyl)propylsulfide (7), at 7 %.
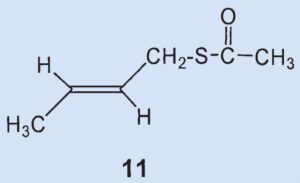
They were able to identify two additional disulfides whose structures were suggested by the mass spectrum: butyl(3-methylbutyl)disulfide (8) and (2-butenyl)butyldisulfide (9). Also, they confirmed that 2-methylquinoline (2) is a component of the secretion and detected the first thioester: thioacetic acid-S-(3-methylbutyl) ester (10).
Thioesters are less volatile than the corresponding thiols. Thioesters adhere to skin and are hydrolyzed over time to form carbonic acid and thiol, making them responsible for the long-lasting effect of skunk secretion.
Results: the secretion contains no bis(2-butenyl)sulfide (3) and no (2-butenyl)mehtyldisulfide (6). The third biggest component is not (6) but (2-butenyl)-propylsulfide (7). Newly discovered components include butyl(3-methylbutyl)disulfide (8), butenyl(2-butyl)-disulfide (9), and thioacetic acid-S(3-methylbutyl) ester (10).
9 1990: The Eighth And Last Attempt to Date
William Wood was the last daring scientist to make an attempt at studying skunk secretion [11]. He carried out GC/MS measurements on fresh secretios, in many cases just a few minutes after it was obtained from the animal. His work confirmed the structures of the two main components, thiols (4) and (5). For the third biggest component, Andersen et al. had proposed the two different structures (6) and (7) in their two studies of 1975 and 1982, respectively.
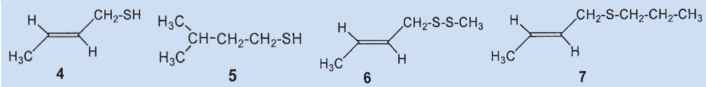
To confirm, Wood synthesized both components and was surprised to determine that neither of the two was present in the secretion. After his spectroscopic studies, the structure of the third biggest component was revealed to be thioacetic acid-S-(2-butenyl) ester (11). This was confirmed by an independent synthesis.
2-Methylquinoline (2), which had been found by Aldrich and Jones in 1897, and the thioacetic acid-S-(3-methylbutyl) ester (10) found by Andersen in 1982 were also confirmed.
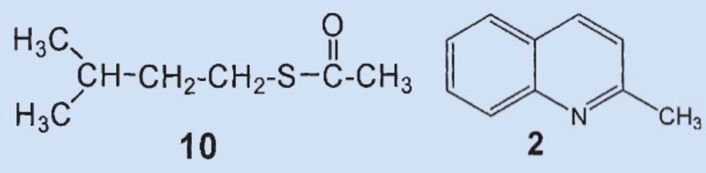
However, Woods was unable to find some of the components that had long been presumed to be present: he did not detect (3), (8), or (9) in the fresh secretion. These products must have been formed by decomposition or reaction with oxygen during separation and/or spectroscopic measurement.
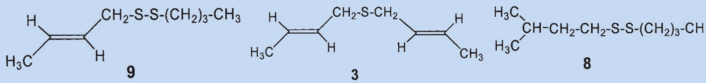
The “isolation” process for sulfide (3) used by Stevens may clarify this: the secretion was stored for several months before being processed. For separation, it was treated for several days with a solution of mercuric chloride in ethanol and the solvent was distilled off after a considerable time. The secretion was then vacuum distilled and then extracted with 7 % hydrochloric acid and then with 10 % “alkali” before a final two vacuum distillations. Stevens’ methods were harsh even for his time and it is amazing that he was able to isolate anything at all in the end.
The disulfide he isolated (3) was most likely formed by oxidation of the (then unknown) primary component (4) by oxygen from the air and subsequent decomposition. Wood was able to detect two new compounds in the skunk secretion: 2-quinolinemethanethiol (12) and thioacetic acid-S-[(2-quinoline)methyl] ester (13).

Results: 2-methylquinoline (2) and thioacetic acid-S-(3-methylbutyl) ester (10) were confirmed to be ingredients of the secretion. The third most prevalent component is neither (6) nor (7), but rather thioacetic acid-S-(2-butenyl) ester (11). The two disulfides (8) and (9) could not be detected in the secretion. 2-Quinolinemethanethiol (12) and thioacetic acid-S-[(2-quinoline)methyl] ester (13) were also identified as components.
10 The State of Affairs
If the results of all the studies are consolidated, the secretion of the striped skunk consists of the primary components (>1 %) listed in Table 2 [1].
Table 2. Primary components of skunk secretion.
| Compound | Fraction in [%] |
|---|---|
| 2-Butene-1-thiol (4) | 38–40 |
| 3-Methyl-1-butanethiol (5) | 18–26 |
| Thioacetic acid-S-(2-butenyl) ester (11) | 12–18 |
| Thioacetic acid-S-(3-methylbutyl) ester (10) | 2–3 |
| 2-Methylquinoline (2) | 4–11 |
| (2-quinoline)methanethiol (12) | 4–12 |
| Thioacetic acid-S-[(2-quinoline)methyl] ester (13) | 1–4 |
11 Quo Vadis, Mephitis Mephitis?
Have we reached the end of chemical research into the skunk, or are there more labile components in fresh skunk spray that decompose quickly in air? What are the decomposition products? Should we next remove the anal glands of an anesthetized skunk and directly inject their contents into a gas chromatograph under an inert gas atmosphere in order to avoid any decomposition? Do all skunks actually smell the same or can we use the chemical composition of their secretion to determine how closely related animals are?
Questions upon questions awaiting an answer. We hope that future researchers looking for action and armed with a powerful deodorant will take up the adorable skunk with new ideas.
To spare researchers whose curiosity has been aroused from the social isolation this work can cause, the American Chemical Society expressly recommends against the use of the commonly suggested but completely ineffective household substances like beer or tomato sauce, suggesting instead the following effective antidote to the strong odor [12]:
|
Dissolve ¼ cup baking soda (bicarbonate of soda, sodium hydrogen carbonate) and 2 teaspoons of liquid soap in 1 liter of 3 % hydrogen peroxide. This solution is used to dampen affected objects (pet fur, skin, and clothing) and left for 5 minutes before rinsing with water. Repeat as needed. |
The elevated pH value of this solution causes the immediate deprotonation of the thiols 4, 5, and 12 to form the water-soluble, non-volatile thiolates. In addition, the thioacetic acid esters 10, 11, and 13 are hydrolytically split. The thiols released in this process are also converted to thiolates. Subsequently, all the thiolates formed in these reactions are quickly oxidized by the hydrogen peroxide to form fully odorless, water-soluble sulfonic acids. This explains the high effectiveness of this solution in fighting skunk odor.
References
[6] Thomas B. Aldrich, Walter Jones, α-Methyl-quinoline as a constituent of the secretion of the anal glands of mephitis mephitica, J. Exp. Med. 1997, 2(4), 439–452. https://doi.org/10.1084/jem.2.4.439
[7] L. Ruzicka, Helv. Chim. Acta 1926, 9, 715 und 1008.
[8] Philip G. Stevens, American Musk. III. The Scent of the Common Skunk, J. Am. Chem. Soc. 1945, 67(3), 407–408. https://doi.org/10.1021/ja01219a015
[9] Kenneth K. Andersen, David T. Bernstein, Some chemical constituents of the scent of the striped skunk (Mephitis mephitis), J. Chem. Ecol. 1975, 1, 493–499. https://doi.org/10.1007/BF00988589
[10] Kenneth K. Andersen, David T. Bernstein, Robert L. Caret, Leo J. Romanczyk, Jr., Chemical constituents of the defensive secretion of the striped skunk (Mephitis mephitis), Tetrahedron 1982, 38(13), 1965–1970. https://doi.org/10.1016/0040-4020(82)80046-X
[11] William F. Wood, New components in defensive secretion of the striped skunk, Mephitis mephitis, J. Chem. Ecol. 1990, 16, 2057–2065. https://doi.org/10.1007/BF01020516
[12] Gross Science, The Chemistry of Skunk Spray – How do you get rid of skunk odor? Don’t use tomato juice… use chemistry!, Public Broadcasting Service, Arlington, VA, USA. (accessed February 26, 2024)
The article has been published in German as:
- Mephitis Mephitis, You Stink!,
Klaus Roth,
Chem. unserer Zeit 2003, 7, 358–361.
https://doi.org/10.1002/ciuz.200390074
and was translated by Caroll Pohl-Ferry.
Who is Nature’s Biggest Stinker? – Part 1
The striped skunk’s secretion is a highly complex natural substance and a masterpiece of sulfur chemistry
See similar articles by Klaus Roth published on ChemistryViews.org




