Laypeople and experts all agree on nature’s biggest stinker: it is Mephitis mephitis, the striped stunk (see Fig. 1).

Figure 1. Mephitis mephitis, the North American striped skunk.
1. The Skunk’s Secretion
Living only in North America, skunks have developed a unique defensive strategy: when threatened, the attacker is given a brief warning as the skunk raises its tail. If the attacker remains oblivious, the skunk sprays them with a few milliliters of a foul-smelling secretion from two anal glands. These encounters are unforgettable for the affected attacker: the secretion induces tears and nausea, clings for a long time, and is repulsive even when highly diluted.
In high concentrations, skunk secretion is also toxic, as demonstrated by a student prank in the USA in 1881. Students from the Virginia Agricultural and Mechanical College in Blacksburg, VA held a classmate captive and forced him to inhale skunk secretion for an extended period of time. The unfortunate student lost consciousness and a doctor was quickly called in to try to resuscitate him – by pouring whiskey down his throat. This Wild West treatment surprisingly helped. The student regained consciousness, suffering from a severe headache. After a night’s sleep, however, all his symptoms disappeared [1].
Given these facts, it is no wonder that few scientists have been bold enough to dare approach the skunk. From the work of these intrepid researchers, we know that the secretion is a highly complex natural substance and a masterpiece of sulfur chemistry.
2. 1862: The First Attempt
It began with Friedrich Wöhler (1800–1882), whose friends from “Neuyork” sent him a sample of the dark yellow, oily, skunk secretion to study. Wöhler handed the “very repulsive smelling” oil off to his Belgian postdoc, T. Swarts for further investigation.
Using distillation, Swarts separated the substance into two sulfur-containing fractions: a colorless, fluid oil (105–110 °C) with a “penetrating odor” and a viscous, yellowish, unpleasant smelling oil (195–200 °C [2]. Steam distillation of the residue remaining after separation of these oils yielded a nitrogen-containing base. The small quantities available only allowed for qualitative chemical investigation.
Wöhler also reported on the physiological effects of skunk secretion: while working, Swarts suffered from severe headaches, his urine smelled of musk, and his “perspiration smelled of the oil for several days.” With these words, Wöhler described the fate of everyone who chose to work in this field of research: they would smell like a skunk for days.
Result: The secretion of skunks is a mixture of substances. It consists of at least one sulfur-containing and one nitrogen-containing compound.
3. 1879: The Second Attempt
The next attempt at the skunk was made by Oskar Löw (1844–1941). However, he was not able to complete his field studies in Texas, USA – more for social reasons than for scientific ones. He reported that, “I had frequent opportunity to collect a sufficient quantity of this secretion to establish its chemical constitution, but all my companions protested against it, declaring the odor which clung to me to be unbearable.”
In his New York laboratory, Löw studied the tiny amounts of secretion he collected despite the protests. His initial investigations confirmed one sulfur-containing and one nitrogen-containing component.
Further studies faltered because of his unsympathetic coworkers [4]: “On my return to New York City, I started a few chemical tests with the little I had collected, when the whole college rose in revolt, shouting, ‘A skunk! There is a skunk here!’ I had to abandon the investigation.”
Result: Wöhler’s findings were confirmed. Active research into skunk secretion also has a social component.
4. 1896: The Third Attempt
In 1896 [4], Thomas Aldrich (1836–1907; John Hopkins University, Baltimore, MD, USA) described the secretion as, “a clear, limpid liquid with a golden-yellow or amber color of a characteristic, penetrating, and most powerful odor and having a specific gravity, at ordinary temperature, less than water (0.939).” [4]
Aldrich focused on the fraction with a boiling point between 100–110 °C. A comparison of the boiling points allowed thiols with three or fewer carbon atoms to be excluded as possible components because their boiling points were too low, and thiols with five or more carbon atoms were ruled out because their boiling points were too high (see Tab. 1).
Table 1. Boiling points of thiols.
| thiol | molecular formula | boiling point [°C] |
| methanethiol | CH4S | 6 |
| ethanethiol | C2H6S | 36 |
| 1-propanethiol | C3H8S | 67 |
| 2-propanethiol | C3H8S | 57–60 |
| 1-butanethiol (1) | C4H10S | 97 |
| 3-methyl-1-butanethiol | C5H12S | 115–120 |
A thiol with four carbon atoms seemed most likely. However, the experimentally determined sulfur contents (35.37 % and 34.98 %) were somewhat low for 1-butanethiol (1) (theoretical for C4H9SH: 35.55 %) and the values for hydrogen and carbon also deviated from theoretically calculated values.
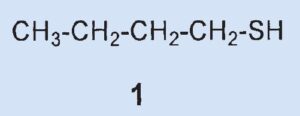 When interpreting his results, Aldrich thus expressed himself very carefully, “The results of these analyses are sufficiently near the theoretical figures when considered together with the boiling point … to convince, I think, the most sceptical that the greater part of this fraction contains one of the butyl mercaptans (butanethiols).”
When interpreting his results, Aldrich thus expressed himself very carefully, “The results of these analyses are sufficiently near the theoretical figures when considered together with the boiling point … to convince, I think, the most sceptical that the greater part of this fraction contains one of the butyl mercaptans (butanethiols).”
In proposing a structure, he speculated, “Primary normal butyl mercaptan (butanethiol) is given as boiling at 97 °C. The boiling point of [the fraction] between 100 and 110 °C could easily be explained if we assume the presence in small quantity of some higher boiling body. This assumption would also explain in general the analytical results. I am inclined to believe in the presence of a higher mercaptan (thiol) rather than a sulfide.”
We must read this carefully. Aldrich frequently alludes to 1-butanethiol (1) in his publication, but never definitively states that it is in fact a component of the secretion. He only says that “it is probable that the secretion contains one of the butanethiols.”
There are four structural isomers of butanethiol: 1-butanethiol, 2-butanethiol, 2-methyl-1-propanethiol, and 2-methyl-2-propanethiol. Despite Aldrich’s careful phrasing, many textbooks state that 1-butanethiol is the main component of skunk secretion. This was shown to be false in 1975, because skunk secretion does not contain any notable amount of 1-butanethiol. Despite this, 1-butanethiol is still falsely described as a component of skunk secretion in many books [5].
Result: The secretion contains a butanethiol, presumably 1-butanethiol (1).
References
[1] William F. Wood, The History of Skunk Defensive Secretion Research, Chem. Educator 1999, 4, 44–50. https://doi.org/10.1007/s00897990286a
[2] T. Swarts, Über das Öl des Stinkthiers, Justus Liebigs Ann. Chem. 1862, 123(2), 266–270. https://doi.org/10.1002/jlac.18621230220
[3] O. Löw, Notizen über das Stinkthier (Mephitis texana), Ärztl. Intell. München 1879, 252–253.
[4] Thomas B. Aldrich, A chemical study of the secretion of the anal glands of mephitis mephitiga (common skunk), with remarks on the physiological properties of this secretion, J. Exp. Med. 1896, 1(2), 323–340. https://doi.org/10.1084%2Fjem.1.2.323
[5] Kenneth K. Andersen, David T. Bernstein, 1-Butanethiol and the striped skunk, J. Chem. Educ. 1978, 55(3), 159. https://doi.org/10.1021/ed055p159
The article has been published in German as:
- Mephitis Mephitis, You Stink!,
Klaus Roth,
Chem. unserer Zeit 2003, 7, 358–361.
https://doi.org/10.1002/ciuz.200390074
and was translated by Caroll Pohl-Ferry.
Who is Nature’s Biggest Stinker? – Part 2
What is in the secretion of skunks? We continue on the fascinating journey of discovery to understand this highly complex natural substance and masterpiece of sulfur chemistry
See similar articles by Klaus Roth published on ChemistryViews.org




