Street, City, Country
 472 Priestley Ave, Northumberland, PA, USA
472 Priestley Ave, Northumberland, PA, USA
Who was there?
Joseph Priestley (1733–1804)
When did he live there?
1794–1804
What is it today?
A museum. The Joseph Priestley House Museum, a National Historic Landmark and National Historic Chemical Landmark, houses Priestley’s house and laboratory. Operated by the Friends of Joseph Priestley House since 2009, it was previously managed by the Pennsylvania Historical and Museum Commission (PHMC).
In the nearby Pond Building visitors can explore the Joseph Priestley Timeline, a series of panels that present his achievements across different periods.
Original building?
Yes.
Architect?
The house was designed by Priestley’s wife Mary.
What is Joseph Priestley known for?
Discovery of Oxygen & Phlogiston Theory
Joseph Priestley is best known for his discovery of oxygen. On Monday, August 1, 1774, in the tiny laboratory of his house in Calne, UK, he heated mercury oxide using a burning lens in the sunlight within a closed apparatus in a pneumatic tub. He obtained a previously unknown gas that supported combustion more effectively than normal air. He called the gas “dephlogisticated” air. However, Priestley failed to recognize the elementary nature of the gas.
He also found that a mouse could live longer in the “new” air than in ordinary air and tried the “new” air himself: “I experienced my chest feeling strangely light and free for a while afterward. Who knows whether this pure air will not one day become a fashionable luxury item? So far, only two mice and I have had the privilege of breathing it in”.
What was remarkable about his discovery was that, unlike Carl Wilhelm Scheele (1742–1786), he was able to publish it promptly. Scheele had likely identified oxygen two years earlier, but his publication was delayed. A Polish alchemist had also described a gas with the properties of oxygen before [3].
Priestley never gave up the phlogiston theory, leaving Antoine Lavoisier (1743–1794) with the privilege of truly understanding oxygen [3]. Priestley concluded the following from his exeriment: The Phlogiston theory says that normal air contains phlogiston. Phlogiston is absorbed by the mercury oxide when it is heated, resulting in mercury (mercury lime + phlogiston) and a type of air that is free of phlogiston. The dephlogisticated air supports combustion because it can absorb phlogiston.
Pneumatic Chemistry At That Time
At that time, English scientists were very successful in gas chemistry, or pneumatic chemistry. Stephen Hales (1677–1761) washed and collected the fumes emitted by heated objects in an inverted vessel by passing them through a kettle filled with water. However, he did not analyze them chemically.
Joseph Black (1728–1799) proved in 1756 that fixed air (CO₂) is different from ordinary air, and demonstrated its production from CaCO₃ in 1756. Priestley’s invention of soda water and his isolation of 20 gases between 1770 and 1800, including sulfur and nitrogen oxides, CO, HCl, O₂, revolutionized gas research [3]. He was an excellent experimentalist, but a weak theorist and a strong nonconformist.

Figure 1. Title page to volume I of Joseph Priestly’s “Experiments and Observations on Different Kinds of Air” (1774). [3]. (public domain)
Life
Joseph Priestley was born on March 13, 1733, in Fieldhead near Leeds, UK, and died on February 6, 1804, in Northumberland, Pennsylvania, USA. The son of a cloth maker, he initially was educated in commerce before studying theology, philosophy, and natural sciences from 1752 to 1755. Priestley worked as an assistant preacher from 1755 to 1760 and later as a language teacher, all while pursuing scientific studies. In 1767, he became a preacher in a poor Leeds parish and conducted gas experiments on the side. By 1773, he was a lecturer and librarian, dedicating himself fully to gas chemistry.
His support for the French Revolution forced him to emigrate to America in 1794, where he became a farmer and continued his scientific work. Priestley supposedly invented the rubber eraser and, through his research on gas solubility, developed a method to carbonate beverages. To isolate water-soluble gases, he developed the pneumatic trough with a mercury barrier (see Fig. 2). He used carbon dioxide captured from his fireplace.
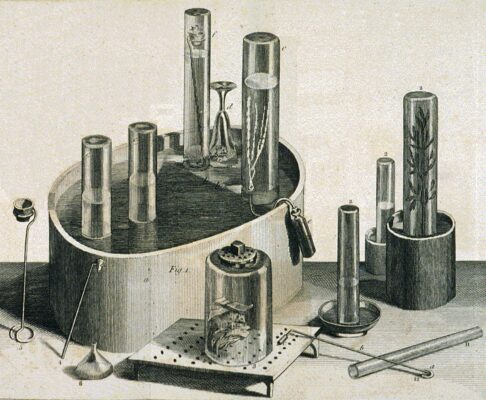
Figure 2. Pneumatic trough, glass collecting cylinders, and other equipment used by Joseph Priestley in his experiments on gases. The right-hand cylinder displays a sprig of mint, demonstrating that plants produce oxygen from carbon dioxide. (public domain)
References/Sources
[1] Bennett R. Willeford, Das Portrait: Joseph Priestley (1733–1804), Chem. Unserer Zeit 1979. https://doi.org/10.1002/ciuz.19790130403
[2] Who Was Joseph Priestley, Priestley House, USA (accessed December 11, 2024)
[3] Experiments and Observations on Different Kinds of Air (1774–86), a six-volume work published by Joseph Priestley
[4] Robert E. Schofield, The Enlightenment of Joseph Priestley: A Study of his Life and Work from 1733 to 1773, University Park: Pennsylvania State University Press, USA, 1997. ISBN 0-271-01662-0
[5] Georg Lockemann, Joseph Priestley In: Das Buch Der Grossen Chemiker, Verlag Chemie GmbH, Band 1, Weinheim, Germany, 1974, S. 263 ff.
→ Back to Overview: Guess the Houses and Molecules




Joseph Priestley lived in Calne, UK in 1784 when he conducted experiments at Bowood House leading to the discovery of Oxygen. This is the home that he lived in when he lived in Calne.
https://www.hamptons.co.uk/properties/20073166/sales/A1NQ500000F2OEWIAR#/
Thank you very much for bringing this to our attention.
As you say, in his laboratory at Bowood House near Calne in Wiltshire, UK, he discovered oxygen in 1774. In June 1773, he was moved with his wife Mary and their three children – Sally, Jos, and William – to the house on The Green in Calne.
In 1775, they relocated to the Parsonage House (now the Old Vicarage on Anchor Road), where their fourth child, Henry, was born on May 24, 1777. In 1780, Priestley moved to Birmingham, UK, to become a minister at the chapel of the New Meeting House.
His support for the French Revolution led to a mob destroying the chapel and setting his house on fire. Continued persecution forced him to emigrate to the USA in 1794.
Reference
Nick Baxter, Joseph Priestley and Calne: A Season of Celebration, Wiltshire and Swindon History Centre, UK, 2024. (accessed December 17, 2024)
My apologies, it was 1774 not 1784, as I previously wrote, when he isolated oxygen at Bowood House in Calne.