Welcome to this week’s research quiz, which focuses on capturing CO2 from the air, making your own reaction vessels for electrosynthesis, and a new method for cleaving alkenes!
Challenge yourself with three questions and see how well you’re staying on top of the latest research news.
 Question
Question
Capturing CO2 from the air offers a promising path toward tackling climate change and reaching carbon neutrality goals. However, it remains challenging. What key properties make the new covalent organic framework, COF-999, stand out as a powerful tool for CO2 capture from open air—earning it praise as one of the most promising direct air capture materials yet, according to the researchers?
See answer
COF-999 has a strong chemically and thermally stable backbone, it shows rapid CO2 uptake and a low regeneration temperature, requires less energy, and can withstand 100 cycles with no loss of capacity, as demonstrated by Omar M. Yaghi, Saudi Arabia, Joachim Sauer, Germany, and colleagues.
 Question
Question
The freely available 3D-printed toolkit Open-ESyn contains downloadable designs for components that are compatible with a commonly used electrosynthesis system. It can make it easier for chemists in the field of organic electrosynthesis to use advanced synthetic techniques and improve their research. Which polymer has suitable properties for use in electrosynthesis and is used to print the components?
See answer
Polypropylene (PP). Open-ESyn enhances IKA’s ElectraSyn 2.0 system with affordable, 3D-printed components such as vials, reaction vessels, electrode clips, lids, and adapters. The toolkit, which was developed by Alastair Lennox, UK, and colleagues, provides customizability without a need for reactors that are entirely designed and machined in-house by researchers. The components increase the range of reactions and parameters that can be used with the system.
 Question
Question
What is triazenolysis? And why should every organic chemist interested in amines know this?
See answer
Triazenolysis is a novel chemical process developed by Mark Gandelman, Israel, and colleagues to cleave carbon–carbon double bonds in alkenes, converting them into amines. Unlike ozonolysis, which transforms alkenes into carbon–oxygen products, triazenolysis uses a nitrogen-rich triazadienium species to produce carbon-nitrogen bonds, forming valuable amines.
This method enables the selective cleavage of both cyclic and acyclic alkenes, offering a practical and efficient way to produce multi-nitrogen compounds from widely available alkene substrates.
The News Behind the Quiz
See the news
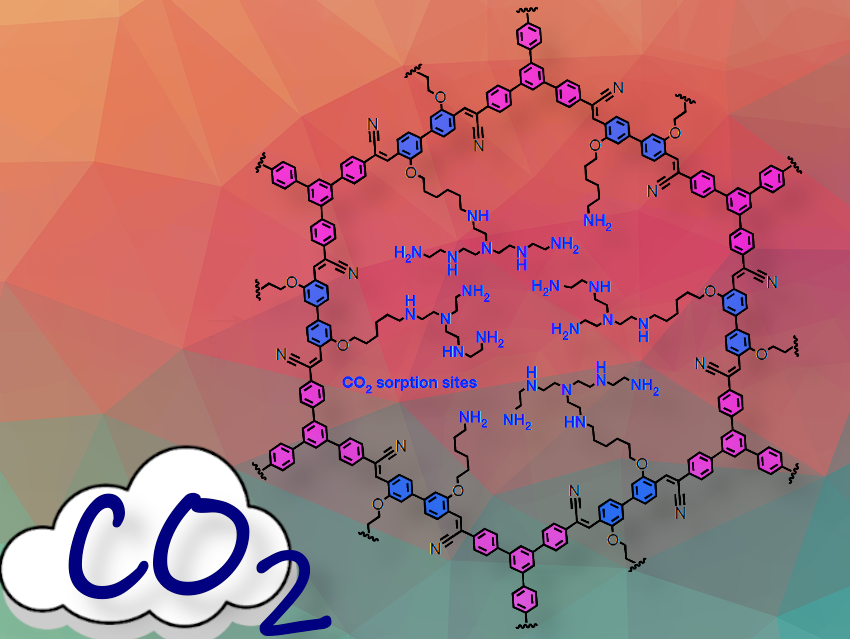 Carbon Dioxide Capture From Open Air
Carbon Dioxide Capture From Open Air
October 24, 2024
COF-999, a stable covalent organic framework, demonstrates exceptional CO2 capture performance from open air, especially in humid conditions
 3D-Printed Toolkit Enhances ElectraSyn Functionality
3D-Printed Toolkit Enhances ElectraSyn Functionality
October 23, 2024
Expanded range and reproducibility for electrosynthesis
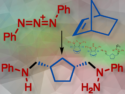 Triazenolysis: A New Approach to Alkene Cleavage for Amine Synthesis
Triazenolysis: A New Approach to Alkene Cleavage for Amine Synthesis
October 21, 2024
A novel method for cleaving alkenes into amines via (3+2) cycloaddition, expanding the scope of alkene transformations



