Welcome to this week’s “Weekly Research News Challenge”!
Answer three questions about last week’s news. This time, the focus is on toxicity checks for cosmetics, the first lanthanide N2 photoswitch, and a a potentially safer and more effective cleavable linker design for targeted drug therapy.
 Question
Question
As an interesting alternative to well-known organic photoswitches, the first lanthanide N2 photoswitch has been developed. Which two states does it switch between?
See answer
The dinitrogen unit in the photoswitch in this study by Enrique R. Batista, Ping Yang, Benjamin L. Davis, and colleagues in the US switches between an side-on and an end-on binding mode (see picture below), i.e., it is either in line with two surrounding metal atoms or perpendicular to them.
The development of such photoswitches based on metal complexes of N2 could open up new possibilities for functional materials, because there are many metal and ligand options to tune their properties.
The News behind the Question
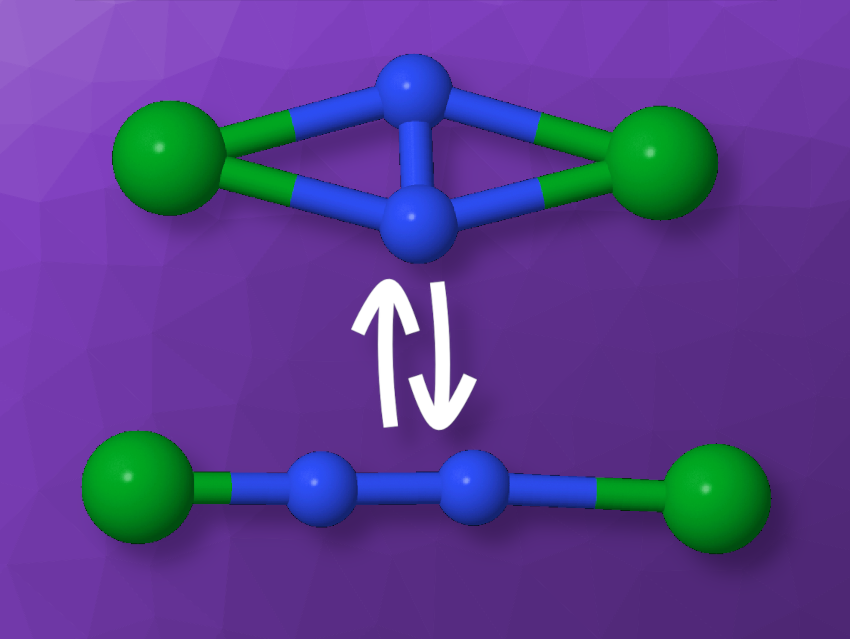 Dinitrogen Turned into a Photoswitch
Dinitrogen Turned into a Photoswitch
November 4, 2024
First lanthanide N2 photoswitch shows isomerization between side-on and end-on coordination
 Question
Question
Why should you always check the ingredient lists of the cosmetics you use every day?
See answer
You should always check the ingredient lists of cosmetics because inspections led by the European Chemicals Agency (ECHA) found that 6 % of nearly 4,500 cosmetic products inspected in 13 European Economic Area (EEA) countries contained hazardous chemicals banned under the Persistent Organic Pollutants (POPs) and REACH regulations.
Restricted substances were found across different types of cosmetic products from various sellers and at all price ranges. The inspections were primarily conducted by checking ingredient lists. Authorities have begun removing non-compliant products from the market but this is likely not an isolated case.
The News behind the Question
 Hazardous Chemicals Found in European Cosmetics
Hazardous Chemicals Found in European Cosmetics
November 05, 2024
Inspections found banned chemicals, such as perfluorononyl dimethicone and D4/D5 siloxanes, in cosmetics like eyeliners, lipliners, and hair conditioners
 Question
Question
Antibody drug conjugates (ADCs) can use different physiological “triggers” to release their drug cargo in a targeted manner. What does the new cleavable linker developed by Utpal Majumder and colleagues in the US use as a trigger to release the drug?
See answer
A natural thiol concentration gradient between cells and the area around them. Traditional linkers often rely on disulfides, whose bond can break under certain conditions. By using thiol gradients, the new linker can be more stable in the bloodstream and only release the drug in the right place.
A linker with a sulfonamide group is used to connect a targeting antibody and a drug “payload”. The different levels of thiols on the inside and the outside of the targeted cells are then used as a trigger: Once inside the targeted cells, the sulfonamide linker is cleaved, resulting in the release of the drug.
This can, for example, be use as a potentially safer and more effective method for delivering cancer treatments.
The News behind the Question
 New Cleavable Linker Design for Targeted Drug Therapy
New Cleavable Linker Design for Targeted Drug Therapy
November 6, 2024
Physiological thiol gradient between extra- and intracellular areas used to trigger novel cleavable linkers
- See more quizzes




