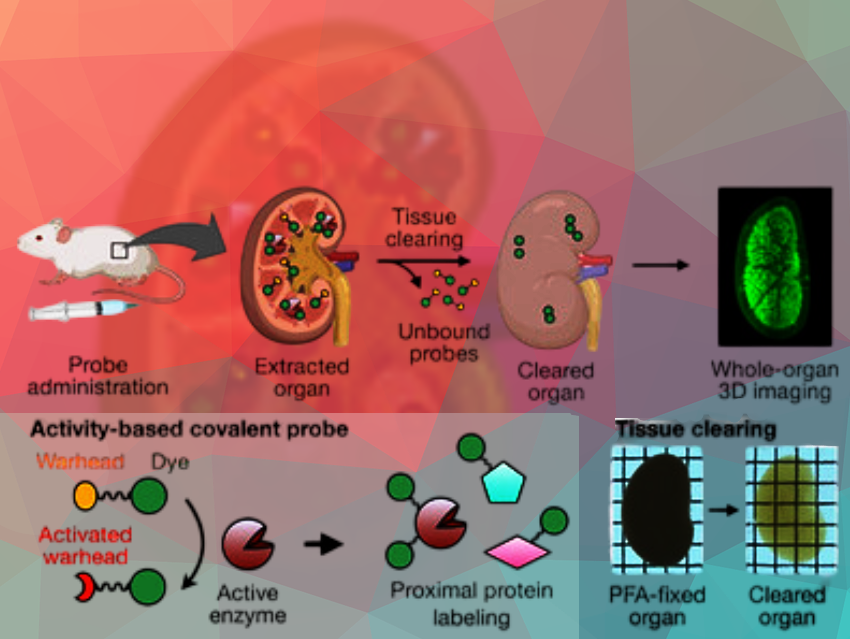
It is now possible to obtain three-dimensional, high-resolution images of enzyme activity in tissue samples or whole organs—thanks to probe molecules that anchor fluorescent dyes within tissue as they are activated by enzymes. The organ being mapped is made transparent by a clearing process. This allowed for visualization of differences in aminopeptidase N activity and the effects of inhibitors in mouse kidneys.
The Need for Spatially Resolved Enzyme Imaging
Enzymes play a crucial role in regulating physiological functions and abnormal enzyme activity is related to a variety of pathological conditions. Enzyme activity varies from organ to organ, as well as within different regions of a single organ. It would thus be informative to obtain precise imaging of enzyme activity in tissues with detailed spatial resolution.
Unfortunately, suitable techniques are lacking. Imaging of enzyme activity is mainly carried out with fluorescence probes. However, fluorescent light barely penetrates tissues, so larger samples like whole organs cannot be mapped in 3D. A process known as clearing could help with this. Clearing is a traditional process by which tissue samples are made highly transparent with different solvents and reagents—while maintaining their structure. However, during the intensive washing, small molecules like fluorescent probes also get washed out of the tissue.
Anchored Fluorescent Probes
A team led by Shinsuke Sando at the University of Tokyo, Japan, has developed a method for imaging the activity of an enzyme in high-resolution 3D within whole, cleared organs. As an example they chose aminopeptidase N (APN), a peptide splitting enzyme that plays an important role in various physiological processes as well as the development of tumors. Their success was due to a specially developed probe molecule that consists of a fluorescent dye (BODIPY), an “anchoring unit”, and an amino acid group (alanine).
If no active APN is present, the probe remains unchanged and is rinsed out during the tissue clearing process. In regions of the organ with active APN, the enzyme splits the amino acid group off the probe, activating the anchoring unit. This attaches to proteins in the immediate area and solidly anchors the fluorescent probe to the surrounding tissue structure so that it is not washed away. This allowed the team to obtain high-resolution, 3D maps of APN activity with fluorescence microscopy in whole mouse kidneys. The researchers were even able to visualize differences in APN activity in individual tubular structures within the kidneys.
Insights from Inhibitor Studies
The team also studied the effects of APN inhibitors. They observed different patterns in the suppression of fluorescence between the experimental antitumor agent actinonin and another APN inhibitor. These differences may result from differences in absorption, metabolism, and/or pharmacokinetics.
According to the researchers, the new imaging technology opens the way to an unbiased evaluation method for drug development that does not overlook tiny phenomena that occur on the cellular level in whole organs.
- Imaging Heterogeneous Patterns of Aminopeptidase N Activity in Hierarchical Tissue Structures Through High-resolution Whole-organ 3D Mapping,
Bo Yi, Hiroyuki Yatabe, Daichi M. Sakamoto, Iori Tamura, Yutaro Saito, Naoki Yamada, Ruki Ashikaga, Masafumi Kuroda, Shimpei I. Kubota, Kazuki Tainaka, Shinsuke Sando,
Angewandte Chemie International Edition 2025.
https://doi.org/10.1002/anie.202504668