Eveline H. Tiekink, Ron Verdijk, Trevor A. Hamlin, F. Matthias Bickelhaupt, Vrije Universiteit Amsterdam,The Netherlands, discuss their recently published study where they used quantum chemistry to show that hydrogen-bonding organocatalysts speed up the Diels–Alder reaction by shifting electron density in a way that reduces repulsion between the reacting molecules. This effect works even with less electronegative groups.
What did you do?
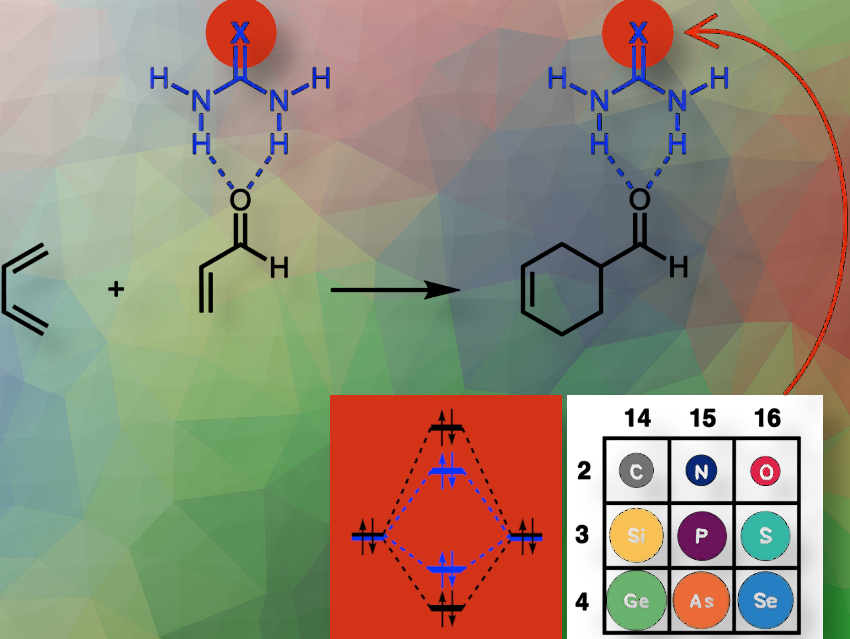
We used computational chemistry to explore a new class of hydrogen bond donor catalysts—derivatives of urea with the general formula (H₂N)₂C=X, where X = O, S, Se, NH, PH, AsH, CH₂, SiH₂, and GeH₂. Through quantum chemical activation strain analyses, we uncovered their ability to catalyze the Diels-Alder reaction, a fundamental transformation in organic synthesis.
This study expands our understanding of catalytic design and challenges conventional wisdom about which molecular motifs can facilitate chemical reactions.
Why are you doing this?
Catalysis is at the heart of chemistry, from drug synthesis to sustainable materials. We hypothesized that these underexplored structural motifs might possess catalytic activity, potentially offering new tools for chemists.
Discovering novel catalytic systems is essential—not only for improving reaction efficiency but also for unlocking new chemical transformations that were previously difficult or impossible.
What is new and cool about it?
The most surprising discovery is that the catalytic effect is not restricted to motifs with highly electronegative elements like oxygen, sulfur, or nitrogen. Even structures like (H₂N)₂C=CH₂ and (H₂N)₂C=SiH₂, which contain carbon and silicon, show catalytic activity.
Some of these catalysts have never been considered before, and yet they show potential to drive chemical reactions.
What are your key findings?
We demonstrated that all tested (H₂N)₂C=X systems, including previously overlooked motifs, can effectively catalyze the Diels-Alder reaction. Notably, (H₂N)₂C=SiH₂, a silicon-containing catalyst, shows promising activity, challenging the assumption that only electronegative elements are effective in this role. This finding broadens the scope of potential hydrogen bond donor catalysts and suggests new directions for both theoretical and experimental research.
What is the longer-term vision for your research?
Our work lays the foundation for experimental chemists to test these newly identified motifs in the lab. If validated, these catalysts could lead to more efficient, selective, and potentially greener synthetic methods.
Looking ahead, we hope this study inspires further research into unconventional hydrogen bond donors, ultimately leading to the development of novel catalytic systems with broad applications in organic synthesis and materials chemistry.
What part of your work was the most challenging?
The challenge has been twofold: The first challenge was to discover and understand why incorporating a more electropositive element X in (H₂N)₂C=X motifs can increase the effective electronegativity of the C=X group, and thus enhance the hydrogen-bonding capability of such species. This challenge had already been mastered by C. Nieuwland, R. Verdijk, C. Fonseca Guerra, and F. M. Bickelhaupt in 2024 [1].
The second challenge was to prove that such enhanced hydrogen-bonding capacity can, in principle, be used to design unorthodox catalysts such as (H₂N)₂C=SiR₂ for Diels-Alder reactions. This is what we did in the present work, which highlights the power of theoretical chemistry to challenge assumptions and reveal hidden opportunities in chemical reactivity and catalysis.
Thank you very much for sharing these insights.
The paper they talked about:
- Organocatalyzed Diels-Alder Reactions: Unexplored Hydrogen Bond Donor Catalysts,
Eveline H. Tiekink, Ron Verdijk, Trevor A. Hamlin, F. Matthias Bickelhaupt,
ChemPlusChem 2025.
https://doi.org/10.1002/cplu.202500081

Eveline H. Tiekink is a PhD student in the Department of Chemistry & Pharmaceutical Sciences at Vrije Universiteit Amsterdam, The Netherlands.

Ron Verdijk has been a Master’s student in the Department of Chemistry & Pharmaceutical Sciences at Vrije Universiteit Amsterdam, The Netherlands. At present, he is a PhD student at the University of Manchester, UK.

Trevor A. Hamlin is an Assistant Professor in the Department of Chemistry & Pharmaceutical Sciences at Vrije Universiteit Amsterdam, The Netherlands.

F. Matthias Bickelhaupt is a Full Professor in the Department of Chemistry & Pharmaceutical Sciences at Vrije Universiteit Amsterdam, The Netherlands; affiliated with the Institute for Molecules and Materials, Radboud University, Nijmegen, The Netherlands; and a Distinguished Visiting Professor in the Department of Chemical Sciences, Faculty of Science, University of Johannesburg, South Africa.
Reference
[1] C. Nieuwland, R. Verdijk, C. Fonseca Guerra, F. M. Bickelhaupt, More Electropositive is More Electronegative: Atom Size Determines C=X Group Electronegativity, Chem. Eur. J. 2024, 30(15), e202304161. https://doi.org/10.1002/chem.202304161