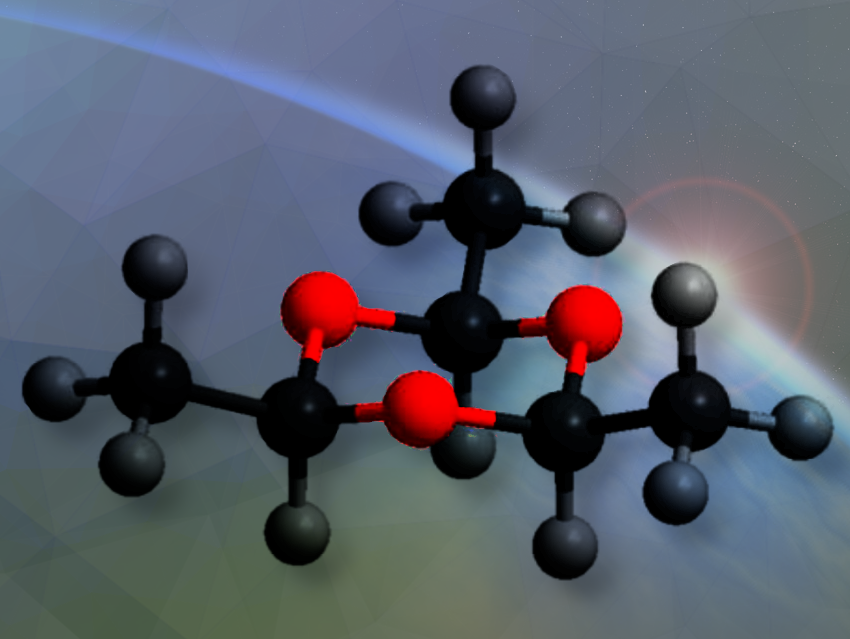
Ralf-Ingo Kaiser, University of Hawaii, Manoa, Honolulu, HI, USA, and colleagues have studied how complex organic molecules like paraldehyde can be synthesized in extraterrestrial ices.
Since its identification in the interstellar medium fifty years ago, acetaldehyde has attracted considerable interest from various scientific communities for its role as a precursor of complex organic molecules, particularly in star-forming regions and interstellar ices. It can react with other simple molecules in low-temperature environments to form biologically relevant compounds, such as acetic acid and pyruvic acid, which are associated with the origin of life. However, the polymerization mechanisms of acetaldehyde under astrophysical conditions are still poorly understood and need further investigation.
What did you do?
We studied the formation mechanisms of complex organic molecules—defined as organic molecules containing six or more atoms in the astronomical context—in interstellar ice analogs.
Using vacuum ultraviolet (VUV) photoionization reflectron time-of-flight mass spectrometry (PI-ReToF-MS) and isotopic substitution experiments, we identified the cyclic acetaldehyde trimer, paraldehyde (C6H12O3), in the gas phase during the temperature-programmed desorption (TPD) of the irradiated acetaldehyde ices, which were exposed to energetic electrons as proxies of galactic cosmic rays (GCRs).
Why are you interested in this?
Acetaldehyde has been detected toward star-forming regions and tentatively identified in interstellar ices. It is a key precursor in the synthesis of astrobiologically relevant molecules linked to the Origins of Life and plays a crucial role in polymerization reactions. However, experimental evidence on its polymerization processes in interstellar ice analogs is still lacking.
Can you explain the advantages of using tunable vacuum ultraviolet PI-ReToF-MS in your study?
We used tunable vacuum ultraviolet PI-ReToF-MS to analyze sublimating molecules from processed acetaldehyde ices during temperature-programmed desorption (TPD).
This approach is an elegant technique to identify gas-phase molecules isomer-selectively through soft photoionization, based on their mass-to-charge ratios and distinct adiabatic ionization energies (IEs). Our results suggest that this method can detect complex organic compounds, including paraldehyde, and offers insights into their formation mechanisms in deep space.
What are your key findings?
We demonstrated the first formation of paraldehyde in low-temperature interstellar analog ices exposed to energetic irradiation as proxies for galactic cosmic rays (GCRs). These findings advance our understanding of how complex organic molecules such as paraldehyde can be synthesized through polymerization reactions in extraterrestrial ices.
What is the longer-term vision for your research?
Since acetaldehyde is ubiquitous throughout the interstellar medium and has been tentatively identified in interstellar ices, our results suggest that paraldehyde may form in acetaldehyde-containing ices in a cold molecular cloud. It could be a suitable candidate for gas-phase observation in star-forming regions using radio telescopes.
Future laboratory experiments could be carried out to identify the acetaldehyde tetramer, metaldehyde (C8H16O4).
What part of your work was the most challenging?
Due to the multiple possible structures of the C6H12O3 isomers, identifying the paraldehyde molecule itself was challenging. In the experiment, a low irradiation dose was designed to minimize sequential reactions in processed acetaldehyde ices.
In addition, in collaboration with Professor Ryan C. Fortenberry from the University of Mississippi, Oxford, USA, the IEs of paraldehyde were calculated using high-level theoretical methods. The tunable vacuum ultraviolet PI-ReToF-MS and isotopic substitution experiments were performed to identify paraldehyde in the gas phase based on the computed IEs and isomer-specific dissociative fragmentation patterns.
Thank you very much for these insights.
The paper they talked about:
- Formation of Paraldehyde (C6H12O3) in Interstellar Analog Ices of Acetaldehyde Exposed to Ionizing Radiation,
Jia Wang, Andrew M. Turner, Joshua H. Marks, Ryan C. Fortenberry, Ralf I. Kaiser,
ChemPhysChem 2024.
https://doi.org/10.1002/cphc.202400837

Ralf-Ingo Kaiser a Professor in the Department of Chemistry and at the W. M. Keck Research Laboratory in Astrochemistry at the University of Hawaii at Manoa, Honolulu, HI 96822, USA.