Burkholderia pseudomallei (BP) bacteria are a threat to human health. These pathogens are difficult to treat, which can cause bad health outcomes and high mortality. Thus, understanding the molecular mechanisms of infection for these bacteria is important, and identifying targets for the development of therapeutics would be useful.
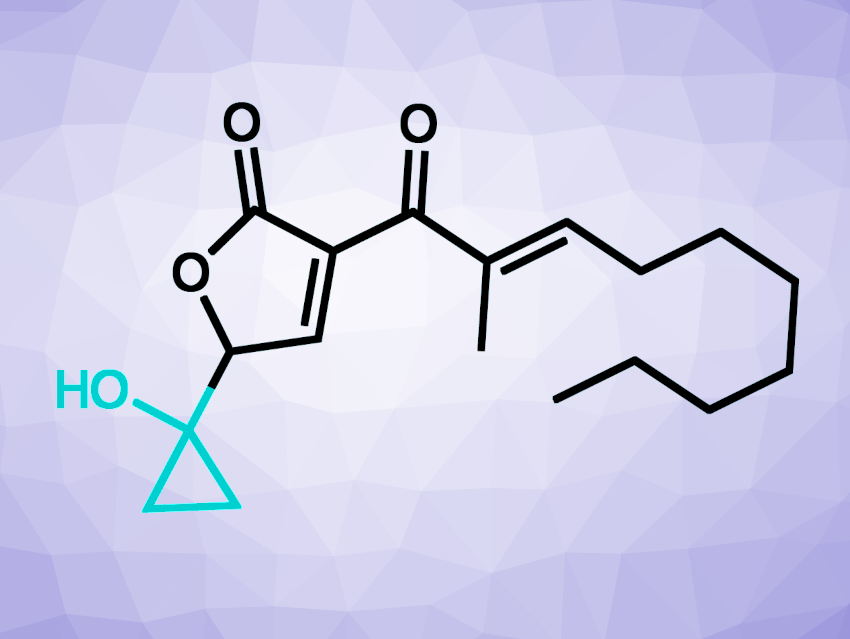
BP group bacteria produce small molecule toxins called malleicyprols (example pictured above) as crucial virulence factors. The bioactivity of the malleicyprols is attributed to their unusual cyclopropanol ring. This could point to possible targets for therapeutics, which could disrupt cyclopropanol formation. However, the biochemical basis of the formation of this group had been unknown so far.
Christian Hertweck, Leibniz Institute for Natural Product Research and Infection Biology – Hans Knöll Institute, Jena, Germany, and Friedrich Schiller University Jena, Michael Groll, Technical University Munich, Garching, Germany, and colleagues have solved the mystery of this cyclopropanol formation. Using a combination of biochemical assays and crystallography, the team identified the enzyme responsible—an elusive cyclopropanol synthase called BurG. The team also studied the associated reaction intermediates and the enzymatic mechanism.
BurG is dependent on the coenzyme NAD+ (nicotinamide adenine dinucleotide). It converts an intermediate that is derived from methionine into a species that contains a cyclopropanol unit (pictured below). This crucial product is then further transformed to give malleicyprols.
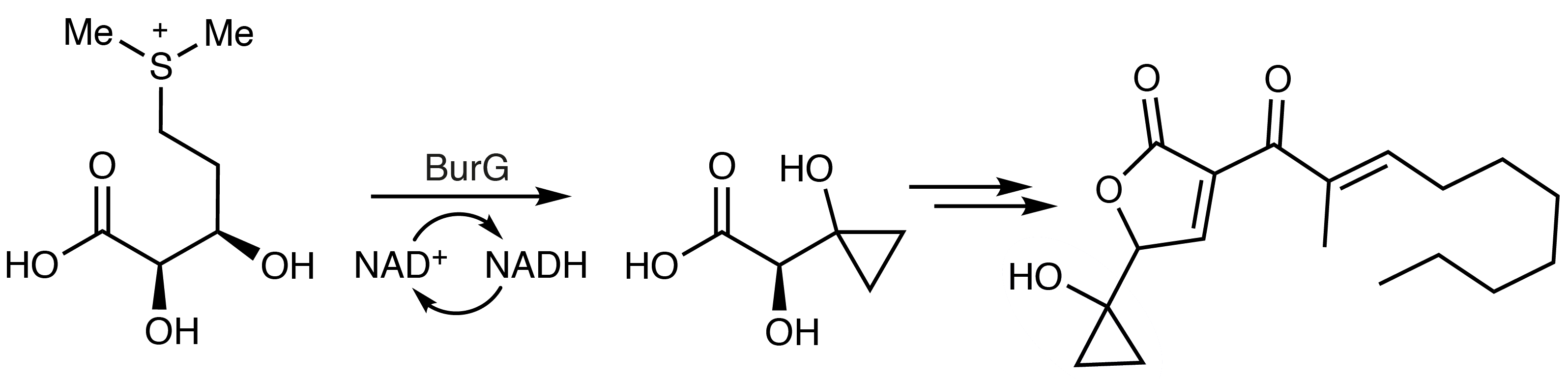
Disarming the BP group pathogens by inhibiting cyclopropanol formation could be a useful anti-virulence strategy. Thus, according to the researchers, BurG is a promising target for the rational design of selective inhibitors to combat BP group infections.
- Pathogenic bacteria remodel central metabolic enzyme to build a cyclopropanol warhead,
Felix Trottmann, Keishi Ishida, Mie Ishida-Ito, Hajo Kries, Michael Groll, Christian Hertweck,
Nat. Chem. 2022.
https://doi.org/10.1038/s41557-022-01005-z