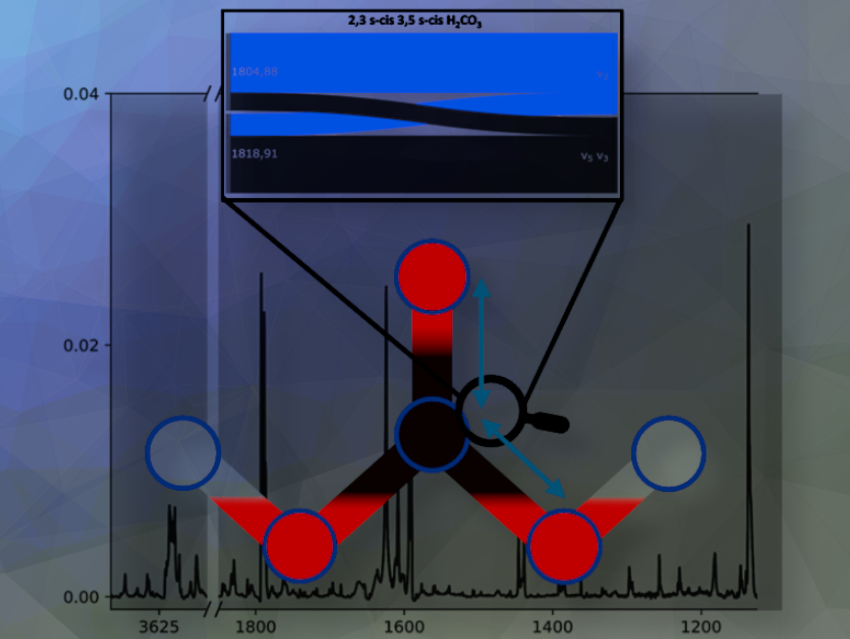
In the minds of chemists as well as in our chemistry textbooks, the idea has taken hold that carbonic acid is too unstable for synthesis. However, over the past 25 years, several syntheses have been discovered in which carbonic acid is produced.
For example, the research group of Erwin Mayer at the University of Innsbruck, Austria, succeeded in synthesizing carbonic acid as a pure crystalline substance in the late 90’s. Shortly afterward, we were able to show that crystalline carbonic acid could even be sublimed without decomposition, making pure gas-phase carbonic acid available for spectroscopic investigation.
Syntheses of this kind have allowed us to investigate the non-rotating single molecule vibrational spectrum of carbonic acid using state-of-the-art computational spectroscopy methods.
Why Carbonic Acid is Highly Interesting
The recent tentative detection of carbonic acid (H₂CO₃) in the gas phase within dense molecular clouds near Sagittarius B2, a large molecular cloud complex in the Milky Way galaxy, has sparked an increase interest in this molecule. The detection of carbonic acid in this region is noteworthy because it challenges the previously held belief that carbonic acid is too unstable to exist in space.
In addition, for Innsbruck, carbonic acid is not a new interest but rather a molecule with a long tradition. Hage, Hallbrucker, and Mayer were the first to succeed in synthesizing carbonic acid as a crystalline pure substance [1]. More than a decade later, Jürgen Bernard used this synthesis method to record non-rotating single-molecule spectra at TU Wien via Matrix-Isolation Infrared (MI-IR) spectra.
The presence of a methyl group in α-carbonic acid was clarified through matrix isolation of sublimed carbonic acid [2,3] combined with theoretical spectroscopy. Solid-state spectra alone [1] could not confirm this due to crystal lattice couplings and the extremely low intensity of the methyl bands. This combined matrix isolation and computational approach revealed that only one polymorph with an undetermined crystal structure (β-carboxylic acid) is known at ambient pressure. The recently determined crystal structure of D2CO3 [4] belongs to a high-pressure polymorp and is not relevant for the detection of solid or gaseous carbonic acid in the Earth’s atmosphere or space.
This work laid the foundation for our current assignment and understanding of carbonic acid’s spectral characteristics.
Most Accurate Computations of the IR Spectrum of Carbonic Acid
Our computations of the IR spectrum of carbonic acid are the most accurate available ones. They demonstrate the very successful progress of theoretical spectroscopy that has been achieved in the last two decades.
Through a combination approach of these highly accurate computations and the experimental spectra of Jürgen Bernard, we were able to reassign the carbonic acid spectrum. We were thus able to assign bands that were completely neglected before.
Previous assignments were relying on an approximation that can lead to misassignments. With our approach, we confirmed some of the previously assigned bands and cleared up some of these misassignments where appropriate. Furthermore, these highly accurate approaches show that in some cases the uniqueness of a band is not given.
In our opinion, with rising computing power and even better methods to describe mode coupling, this problem will become even more acute in the coming years, and will pose a problem for the spectroscopy community.
We believe that the combined strategy of experiment and theory we follow in this work, as well as in previous similar studies on other molecules, enables more reliable assignments of ínfrared spectra and will be the direction where the field of infrared spectroscopy is heading. This is essential for fields that heavily rely on spectroscopy, such as astrochemistry.
Research Challenges
The first most challenging part of a spectroscopic work is being confident with your assignment. As we have explained in our article, multiple strategies have to be combined to foster the argumentation of the assignment.
Today, we are in the lucky position that we can use high-performance computers to calculate the spectra with unprecedented accuracy. However, these kind of calculations must be set up very thoroughly and well thought out, which is probably the second most challenging part of our work.
Source
- Solving the puzzle of the carbonic acid vibrational spectrum – An anharmonic story,
Jonas Schlagin, Dennis F. Dinu, Jürgen Bernard, Thomas Loerting, Hinrich Grothe, Klaus Liedl,
ChemPhysChem 2024.
https://doi.org/10.1002/cphc.202400274
The work was conducted as a collaboration of three research groups, in the framework of the research focus area “Functional Materials Science (FunMAT)” at the University of Innsbruck (UIBK), in collaboration with the Institute of Materials Chemistry at TU Wien.
References
[1] W. Hage, A. Hallbrucker, E. Mayer, Carbonic acid: synthesis by protonation of bicarbonate and FTIR spectroscopic characterization via a new cryogenic technique, Journal of the American Chemical Society 1993, 115, 8427–8431. https://doi.org/10.1021/ja00071a061
[2] Jürgen Bernard, Eva-Maria Köck, Roland G. Huber, Klaus R. Liedl, Ludwig Call, Robert Schlögl, Hinrich Grothe, Thomas Loerting, Carbonic acid monoethyl ester as a pure solid and its conformational isomerism in the gas-phase, RSC Adv. 2017. https://doi.org/10.1039/C7RA02792C
[3] Eva-Maria Köck, Jürgen Bernard, Dr. Maren Podewitz, Dennis F. Dinu, Roland G. Huber, Klaus R. Liedl, Hinrich Grothe, Erminald Bertel, Robert Schlögl, Thomas Loerting, Alpha-Carbonic Acid Revisited: Carbonic Acid Monomethyl Ester as a Solid and its Conformational Isomerism in the Gas Phase, Chem. Eur. J. 2019. https://doi.org/10.1002/chem.201904142
[4] Sebastian Benz, Da Chen, Andreas Möller, Michael Hofmann, David Schnieders, Richard Dronskowski, The Crystal Structure of Carbonic Acid, Inorganics 2022, 10(9), 132. https://doi.org/10.3390/inorganics10090132
Authors
Jonas Schlagin, Dennis F Dinu, Jürgen Bernard, Thomas Loerting, and Klaus Liedl from the University of Austria, and Hinrich Grothe from TU Wien, Austria