Cysteine is often used as a site for the functionalization of proteins or peptides. This type of functionalization, or bioconjugation, is useful, e.g., in medicinal chemistry. Bioconjugation reagents need to work well under mild reaction conditions that are compatible with the substrates and show good selectivities. Using stoichiometric organometallic reagents can be promising in this context, because it simplifies the reaction conditions compared with similar transition-metal-catalyzed reactions.
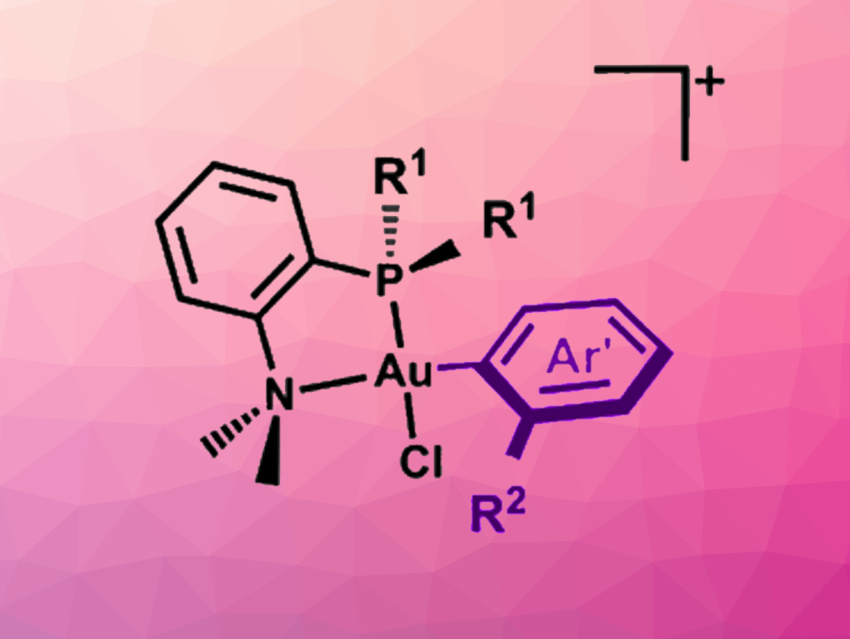
K. N. Houk, Heather D. Maynard, Alexander M. Spokoyny, University of California, Los Angeles, USA, and colleagues have developed an Au(III)-based cysteine arylation reagent that enables an ultrafast reaction. The team prepared gold(III) complexes with different 2-phosphino-N,N-dimethylaniline ligands (general structure pictured) and tested them in cysteine arylation reactions. The team found that the structural variations allowed them to tune the cysteine arylation kinetics.
For the desired ultrafast reactions, dicyclohexyl phosphino-containing reagents (R1 = Cy) performed very well. According to the researchers, this reagent provides the fastest rate of bioconjugation to an amino acid by an organometallic oxidative addition (OA) complex measured to date. The approach allows for bioconjugations at very low concentrations. In addition, reagents with two gold atoms can be used to introduce aryl linkers between cysteines in separate proteins/peptides and build biomolecular heterostructures.
- Ultrafast Au(III)-Mediated Arylation of Cysteine,
Evan A. Doud, James A. R. Tilden, Joseph W. Treacy, Elaine Y. Chao, Hayden R. Montgomery, Grace E. Kunkel, Eileen J. Olivares, Nima Adhami, Tyler A. Kerr, Yu Chen, Arnold L. Rheingold, Joseph A. Loo, Christopher G. Frost, K. N. Houk, Heather D. Maynard, Alexander M. Spokoyny,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.3c12170