Aromatic subunits can be used to build organic compounds with a variety of three-dimensional shapes, such as sphere-like fullerenes, bowl-shaped corannulenes, screw-shaped helicenes, or belt-shaped cycloparaphenylenes. Many of these species can be prepared from separate building blocks using a bottom-up approach. However, some shapes can be challenging to access this way. Macrocycles made from aromatic building blocks with a twisted, figure-eight-like conformation, for example, can be difficult to synthesize using a bottom-up approach due to possible side reactions and a need for multi-step transformations. Such figure-eight-shaped structures are chiral with a D2-symmetry and can show, for example, efficient circularly polarized luminescence (CPL).
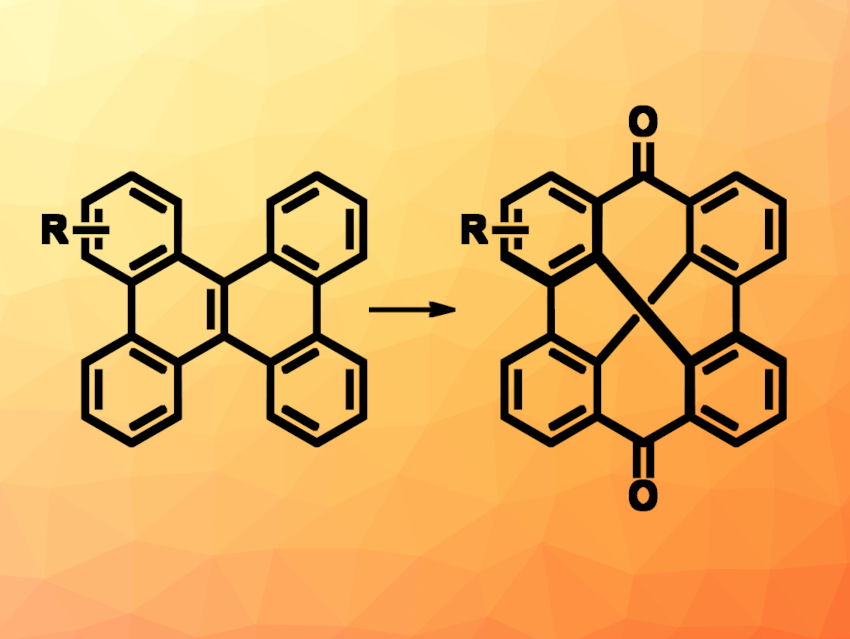
Norihito Fukui, Nagoya University, Japan, and Japan Science and Technology Agency (JST), Kawaguchi, and colleagues have developed an alternative synthetic approach to a figure-eight-shaped aromatic macrocycle (pictured). Instead of a bottom-up synthesis, the team used the oxidative cleavage of an internal double bond of a polycyclic aromatic hydrocarbon (PAH) to create the desired product. They started from, e.g., dibenzo[g,p]chrysene (DBC, pictured above on the left, R = = H), which was first cis-dihydroxylated using a ruthenium-catalyzed reaction with NaIO4. The resulting internally dihydroxy-substituted intermediate then underwent an organoiodine(III)-catalyzed oxidative glycol cleavage to break the central bond and obtain the desired cyclobisbiphenylenecarbonyl (CBBC, pictured above on the right, R = H).
The method was extended to a range of substituted derivatives, and could also be scaled up to the gram scale. From the racemic dihydroxy-substituted intermediate, the team was able to perform an oxidative kinetic resolution in the presence of a chiral iodoarene to obtain a single enantiomer of the figure-eight-shaped macrocycle, which shows efficient CPL. When the developed transformation was performed using larger PAHs with multiple internal C=C bonds, products with multiple figure-eight-shaped subunits could be obtained. Overall, the work provides products that could be useful for the preparation of new functional materials.
- Inner-Bond-Cleavage Approach to Figure-Eight Macrocycles from Planar Aromatic Hydrocarbons,
Reiji Yoshina, Junichiro Hirano, Emiko Nishimoto, Yuki Sakamoto, Keita Tajima, Shunsuke Minabe, Muhammet Uyanik, Kazuaki Ishihara, Tomoyuki Ikai, Eiji Yashima, Takuya Omine, Fumitaka Ishiwari, Akinori Saeki, Jinseok Kim, Juwon Oh, Dongho Kim, Guanting Liu, Takuma Yasuda, Hiroshi Shinokubo, Norihito Fukui,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.4c07985