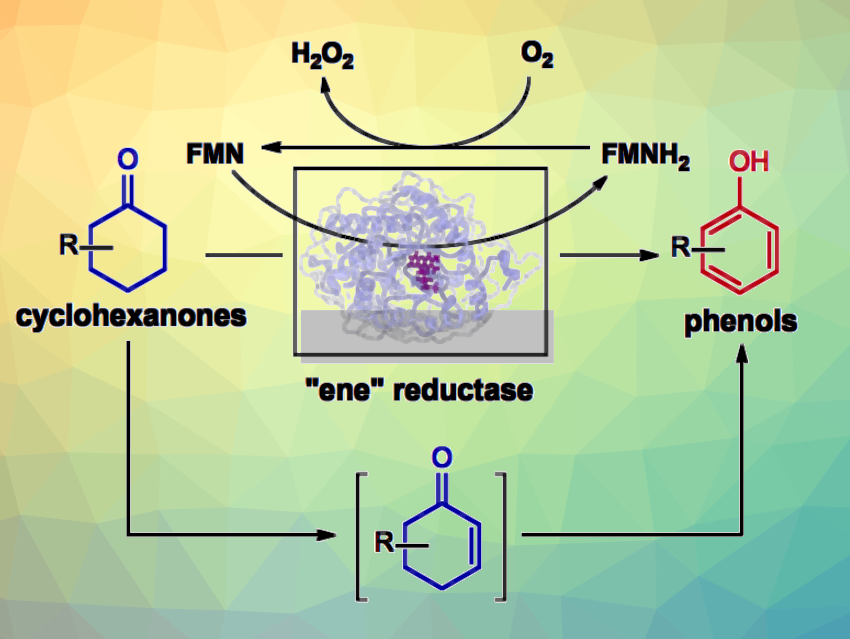
Phenol derivatives are useful building blocks in organic synthesis. They are widely used across the chemical industry and important, e.g., in pharmaceutical chemistry. Substituted phenols are often prepared via electrophilic aromatic substitution reactions, which can suffer from limited regioselectivity. Cyclohexanones can be useful alternative substrates, leading to phenols via aromatization.
Yujing Hu, Kai Guo, Nanjing Tech University, China, and colleagues have developed a biocatalytic method for the aromatization of cyclohexanones that uses an ene-reductase enzyme (TsER from from thermophilic Thermus scotoductus bacteria), that can efficiently catalyze this transformation. The team used both wild-type and engineered strains of the enzyme to synthesize a series of ortho-, meta-, and para-substituted phenolic compounds. The transformation involves the dehydrogenation of two saturated C–C bonds within the cyclohexanone ring and uses molecular oxygen from the air to drive the reaction cycle.
The enzyme can catalyze the reaction in the absence of NAD(P)H (nicotinamide adenine dinucleotide/nicotinamide adenine dinucleotide phosphate). It uses reduced flavin mononucleotide (FMNH2) as a hydrogen donor instead. The method can be readily scaled up to the mmol scale. According to the team, the work provides an efficient, clean, and mild approach to the synthesis of valuable aromatic compounds.
- Ene‐Reductase‐Catalyzed Aromatization of Simple Cyclohexanones to Phenols,
Jie Chen, Shaofang Qi, Zhiguo Wang, Liran Hu, Jialing Liu, Guixiang Huang, Yongzhen Peng, Zheng Fang, Qi Wu, Yujing Hu, Kai Guo,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202408359