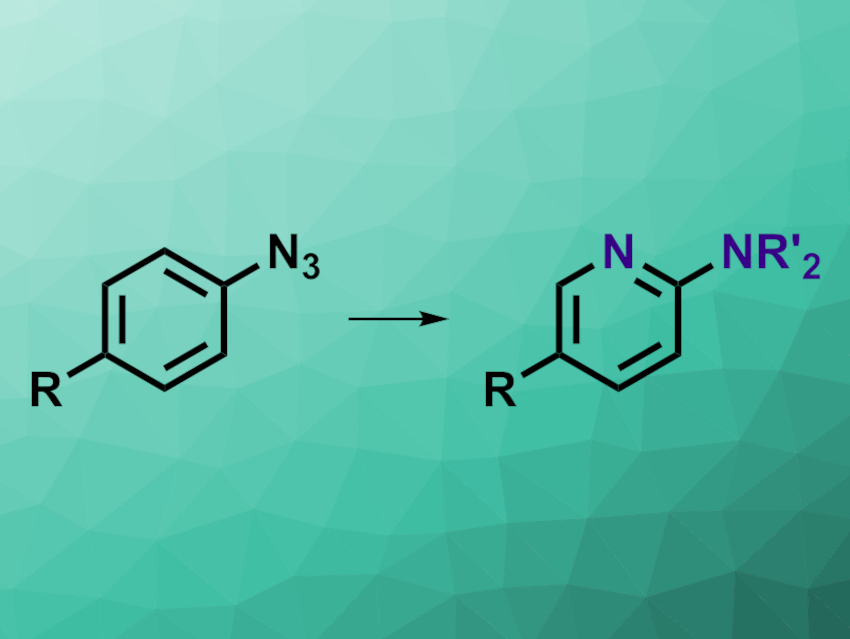
Functionalizing arenes is commonplace in organic chemistry. Converting the arene core into a pyridine, in contrast, is much more of a challenge. This type of reaction could be useful, for example, in pharmaceutical chemistry, where exchanging a carbon atom for a nitrogen atom can help to tune the properties and activities of compounds. So far, this usually requires an entirely new synthesis in the case of exchanging a benzene unit for a pyridine. The direct conversion of benzene into pyridine via a carbon-to-nitrogen exchange had remained very challenging.
Sajan C. Patel and Noah Z. Burns, Stanford University, CA, USA, have developed a method for the conversion of aryl azides to 2-aminopyridines (pictured) in a one-pot process. The team used a variety of substituted aryl azides as substrates and first reacted them with different secondary or primary amines in tetrahydrofuran (THF) under blue LED light, leading to a ring expansion under nitrogen insertion. In the second step of the one-pot process, the researchers added acenaphthylene as a photosensitizer and oxygen, while keeping the blue light irradiation and performing a solvent swap to MeOH. Under these conditions, a ring contraction gives the desired pyridine core.
The 2-aminopyridine products were obtained in low to moderate yields. The team proposes a mechanism that involves the formation of a nitrene from the azide, which inserts into an arene π-bond to give a 2H-azirine. This species is converted to an electrophilic seven-membered ring that reacts with the amine. The resulting intermediate reacts with singlet oxygen, possibly forming a peroxide-substituted derivative. This intermediate can undergo a cyclization to give a cyclopropyl-fused dihydropyridine. A cyclopropane ring opening and loss of hydroxide are then followed by a methanol-mediated deformylation to give the desired 2-aminopyridine and methyl formate.
- Conversion of Aryl Azides to Aminopyridines,
Sajan C. Patel, Noah Z. Burns,
J. Am. Chem. Soc. 2022.
https://doi.org/10.1021/jacs.2c08464