Cláudio M. Nunes, University of Coimbra, Portugal, and Peter R. Schreiner, Justus Liebig University, Giessen, Germany, discuss their recently published work on controlling quantum mechanical tunneling (QMT) reactivity by modifying electronic substituents on a model system, and why they believe QMT must be incorporated into general chemistry curricula.
What did you do?
Controlling quantum mechanical tunneling (QMT) reactivity is highly challenging and not well studied, as it requires strategies different from the established ways of describing chemical reactions. In our study, we explored how QMT reactivity can be controlled by modifying electronic substituents on a model system.
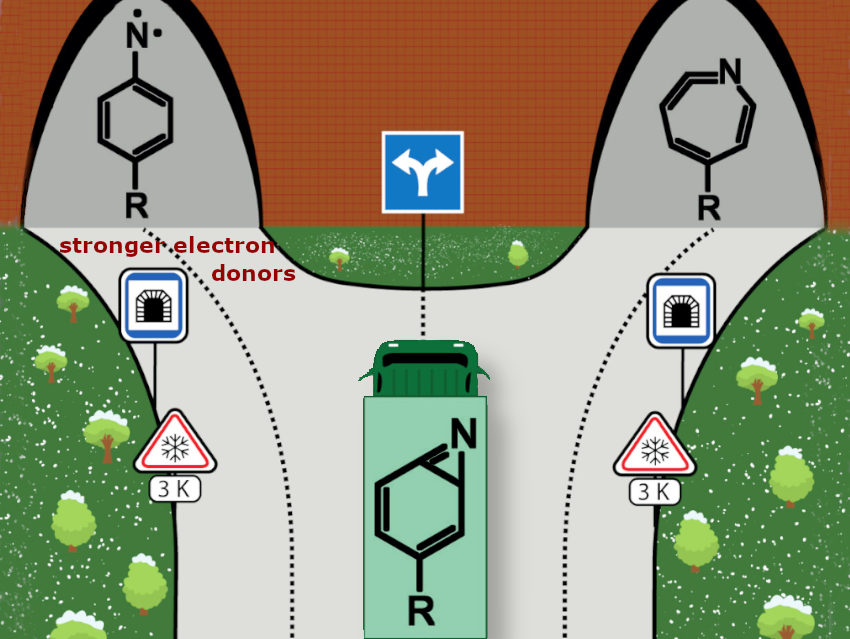
We used substituted benzazirines, which are unique compounds that react through two competing QMT pathways. Three new derivatives (R = OH, N(CH3)2, and N(CH2)4) with increasingly stronger electron-donating para-substituents were studied in argon matrices at very low temperatures (3 K).
Remarkably, each derivative showed different QMT reactivity, resulting in different product distributions. As the electron-donating ability of the substituent increased, ring-opening to a nitrene began to compete with and eventually dominate the alternative pathway, namely ring-expansion to a ketenimine.
Our computational analyses highlight how small adjustments to electronic properties can precisely control the outcomes of QMT reactions.
Why are you interested in this research?
QMT enables entirely new chemical reactivity that can also lead to new synthetic pathways or even products.
What is especially fascinating for you about QMT?
The fascination arises from the fact that most chemists have not been and largely still are not familiar with QMT reactivity, even though nature uses it routinely. This opens the door to a much deeper understanding of chemistry and biology.
What are your key findings?
Whereas we had introduced the notion of “tunneling control of chemical reactions” next to kinetic and thermodynamic control, we are now making the first strides into “controlling tunneling for chemical reactions”. The present paper demonstrates that we can use our familiar concepts of electron-donor and electron-acceptor groups to steer QMT-dominated reactions in a particular direction.
What is the longer-term vision for your research?
The external control of QMT reactions—the nudging of potential energy hypersurfaces—opens new possibilities to control chemical reactions and save energy at the same time because potentially high reaction barriers do not necessarily have to be overcome if a QMT process takes over.
What part of your work was the most challenging?
Deciphering QMT effects remains a challenging task and is best conducted at very low temperatures, where thermal over-the-barrier activation is excluded. Generating target systems to understand and develop the control of tunneling in chemical reactions—specifically involving two competing QMT pathways—proved particularly challenging, especially considering that such systems must exhibit QMT kinetics within a measurable time frame of a few minutes to a few days to allow our spectroscopic measurement.
Anything else you would like to add?
We hope that many chemists pick up on the notion of QMT in chemistry. It is imperative that this phenomenon is included in general chemistry teaching curricula.
Thank you very much for sharing these insights.
The paper they talked about:
- Competitive Heavy-Atom Tunneling Reactions Controlled through Electronic Effects,
José P. L. Roque, Cláudio M. Nunes, Fumito Saito, Bastian Bernhardt, Rui Fausto, Peter R. Schreiner,
ChemistryEurope 2024.
https://doi.org/10.1002/ceur.202400060

Peter R. Schreiner is a Professor at the Institute of Organic Chemistry at the Justus Liebig University (JLU), Giessen, Germany. (Photo: © JLU/David Ausserhofer)

Cláudio M. Nunes is a Professor at the Department of Chemistry of the University of Coimbra, Portugal. (Photo: © Cláudio Manaia Nunes)