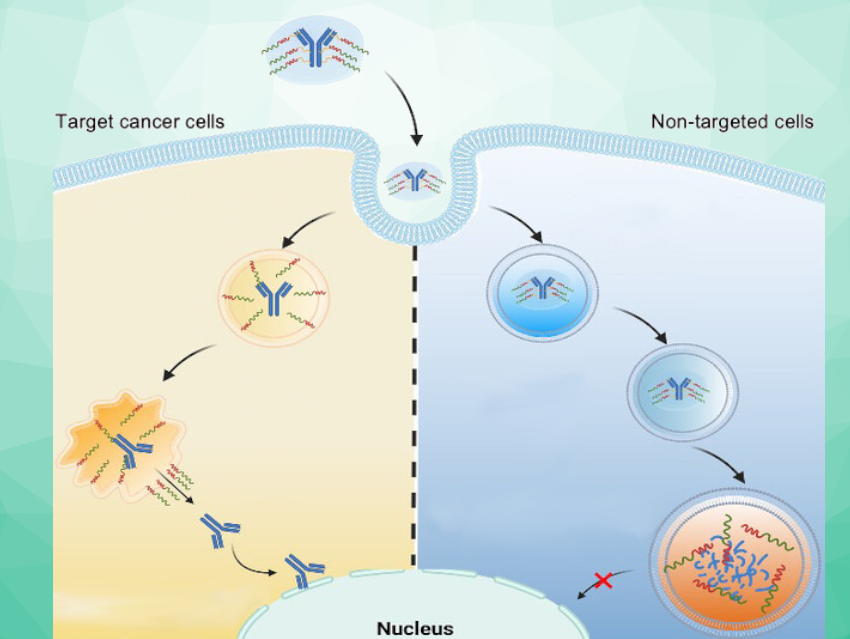
Protein-based drugs must be transported into cells in a way that prevents their immediate degradation. A new approach is intended to ensure that they remain intact only in certain cells, such as cancer cells. Kazunori Kataoka, Innovation Center of Nanomedicine (iCONM), Kawasaki Institute of Industrial Promotion, Japan, Horacio Cabral, The University of Tokyo, Japan, and colleagues have developed a nanocarrier that can “escape” from endosomes (small vesicles surrounded by a membrane) before its cargo is destroyed there. This ability to escape is only triggered within the endosomes of certain tumor cells.
Cellular Uptake and Escaping Enzymatic Breakdown
The uptake of nanocarriers into cells occurs by endocytosis: When a nanocarrier lands on the cell surface, the cell membrane folds in and encloses it in a kind of “bubble”, an endosome, which then drifts into the cell interior. In its late phase, the endosome merges with lysosomes (another type of membrane-bound cell organelle) that contain enzymes, forming an endolysosome. Within this structure, the enzymes break down both material from the body and foreign material.
A protein-based drug can only become active if it “escapes” the endolysosome before being broken down. This is known as “endosomal escape”. Some nanocarriers can open the endo/lysosomal membrane, and thus, have endosomal escape ability.
Targeted Delivery by Activated Escape
The team aimed to take this a step further by producing nanocarriers for which endosomal escape is only triggered when they enter very specific cells, such as tumor cells. This would protect healthy cells. The researchers exploit the fact that different types of cells have very different endolysosomal enzyme activities. For example, the activity of the protease cathepsin B (CTSB) is especially high in cancer cells.
With the use of special fluorescence probe molecules, the team studied CTSB activity and protein degradation in endosomes. They determined that in cancer cells with highly acidic endosomes, CTSB activity is already very high in their early phase—i.e., before protein degradation ramps up. The researchers take advantage of this time window by using nanocarriers whose endosomal escape ability is triggered by the CTSB in cancer cells.
Antibody-Loaded Polymeric Nanocarriers
The team used poly(ethylene glycol)-based polymers with diaminoethane groups capable of “tearing open” the endo/lysosomal membranes. Using a linker, they then attached these polymers to antibodies to act as a model for a protein drug. The resulting nanocarrier shields the “membrane-tearing tools” so that they are initially inactive.
The team used the anti-nuclear pore complex antibody (anti-NPC) as the model protein. The “membrane-tearing” polymer, poly(ethylene glycol)-poly{N-[N’-(2-amino-ethyl)-2-aminoethyl]aspartamide} (PEG-pAsp(DET), active part pictured in red) was bound to the antibodies via a CTSB-sensitive linker (val-cit, pictured in yellow) to form the desired nanocarrier.
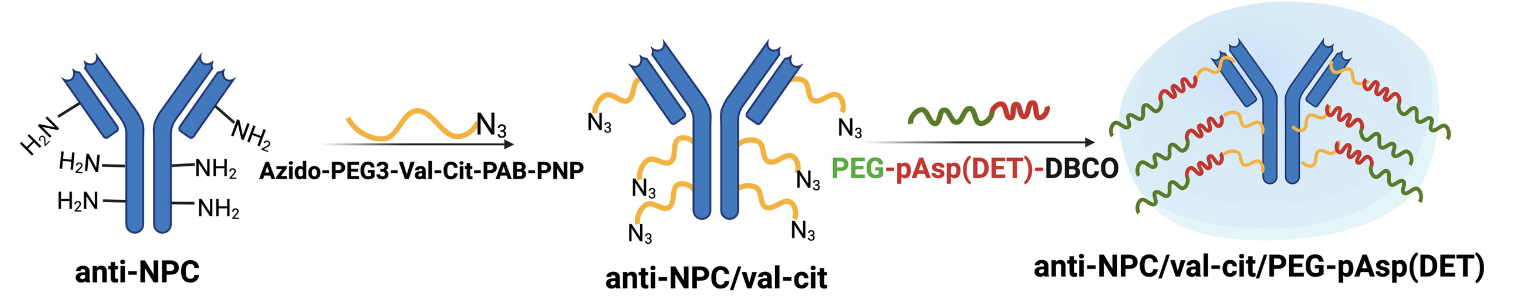
The linker is designed to be split by the CTSB in the endolysosomes. This separates the cargo from the carrier, activating the tearing tools. They open the endo/lysosomal membrane and release intact antibodies into the cell interior—but only in tumor cells that have elevated endosomal CTSB activity.
Overall, this method could represent a new strategy for the cell-specific release of drugs through stimulus-responsive nanocarriers with controlled endosomal escape.
- Selective Intracellular Delivery of Antibodies in Cancer Cells with Nanocarriers Sensing Endo/Lysosomal Enzymatic Activity,
Pengwen Chen, Wenqian Yang, Yuki Mochida, Shangwei Li, Taehun Hong, Hiroaki Kinoh, Kazunori Kataoka, Horacio Cabral,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202317817