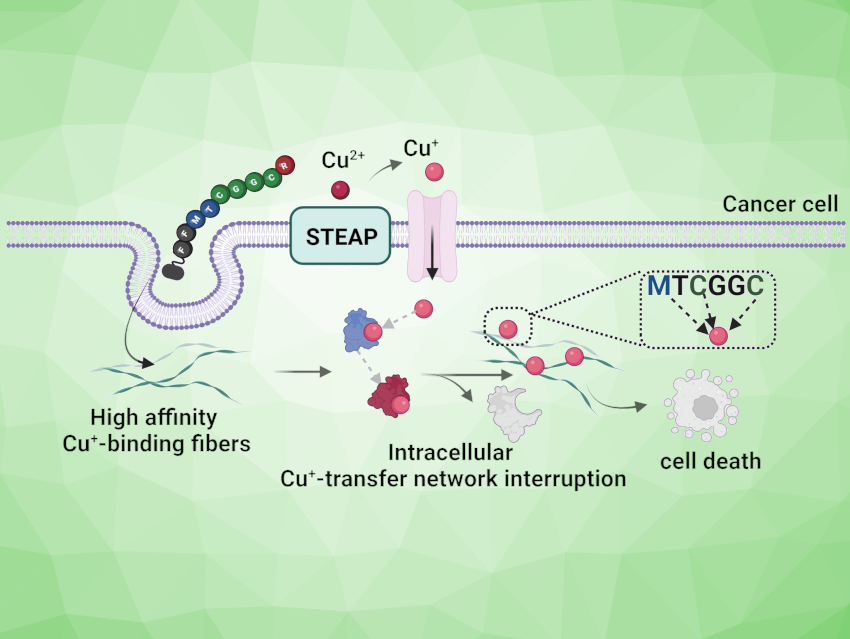
While toxic in high concentrations, copper is essential to life as a trace element. Many tumors require significantly more copper than healthy cells for growth—a possible new point of attack for cancer treatment. Jianghong Rao, Stanford University, CA, USA, David Y. W. Ng, Max Planck Institute for Polymer Research, Mainz, Germany, and colleagues have introduced a novel method by which copper is effectively removed from tumor cells, killing them.
Cellular Copper Equilibrium
Copper is an essential cofactor for a variety of enzymes that play a role in the growth and development of cells. For example, copper ions are involved in antioxidant defense. Cells very strictly regulate the concentration and availability of copper ions. On the one hand, enough copper ions must be on hand; on the other, the concentration of free copper ions in the cytoplasm must be kept very low to avoid undesired side effects.
Extracellular, doubly charged copper ions are reduced to singly charged copper, transported into the cell, stored in pools, and transferred to the biomolecules that require them on demand. To maintain the cellular copper equilibrium (homeostasis), cells have developed clever trafficking systems that use a variety of transporters, ligands, chaperones (proteins that help other complex proteins to fold correctly), and co-chaperones.
Copper in Cancer Cells
Because cancer cells grow and multiply much more rapidly, they have a significantly higher need for copper ions. Restricting their access to copper ions could be a new therapeutic approach. The problem is that it has so far not been possible to develop drugs that bind copper ions with sufficient affinity to “take them away” from copper-binding biomolecules.
The team successfully developed such a system. At the heart of their system are the copper-binding domains of the chaperone Atox1. The team attached a component to this peptide that promotes its uptake into tumor cells. An additional component ensures that the individual peptide molecules aggregate into nanofibers once they are inside the tumor cells. In this form, the fiber surfaces have many copper-binding sites in the right spatial orientation to be able to grasp copper ions with thiol groups in a chelate complex.
Peptide Nanofibers
The affinity of these nanofibers for copper is so high that they also grab onto copper ions in the presence of copper-binding biomolecules. This drains the copper pools in the cells and deactivates the biomolecules that require copper. As a consequence, the redox equilibrium of the tumor cell is disturbed, leading to an increase in oxidative stress, which kills the tumor cell. In experiments carried out on cell cultures under special conditions, over 85 % of a breast cancer cell culture died off after 72 h while no cytotoxicity was observed for a healthy cell culture.
The research team hopes that some years in the future, these fundamental experiments will perhaps result in the development of useful methods for treating cancer.
- Chaperone‐Derived Copper(I)‐Binding Peptide Nanofibers Disrupt Copper Homeostasis in Cancer Cells,
M. T. Jeena, Julian Link, Jian Zhang, Iain Harley, Petri Turunen, Robert Graf, Manfred Wagner, Luis Andre Baptista, Hendrik R. A. Jonker, Liyang Cui, Ingo Lieberwirth, Katharina Landfester, Jianghong Rao, David Y. W. Ng, Tanja Weil,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202412477