Antitumor properties have been observed for some cannabinoids. However, the exact mechanisms leading to cancer cell death are not always known. The synthetic compound WIN 55,212-2, for example, is a potent agonist at both cannabinoid type 1 (CB1) and cannabinoid type 2 (CB2) receptors. The compound’s structure features a fused tricyclic core. SDB-001 is another non-selective agonist at both cannabinoid receptors and contains an adamantyl carboxamide unit.
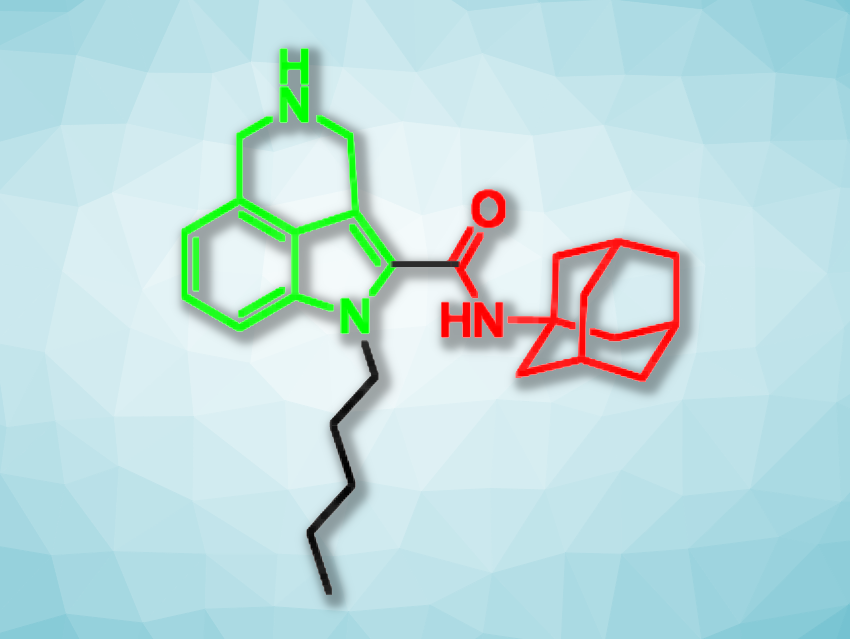
Hendra Gunosewoyo, Curtin University, Bentley, Australia, and colleagues have used these two compounds as inspiration and synthesized six new 3,4-fused tricyclic indole-2-carboxamides (example pictured, tricyclic unit in green, adamantyl carboxamide unit in red). The team started from commercially available methyl indole-4-carboxylate, which was first converted to an aldehyde and then to an oxime. The oxime was reduced, and a tricyclic amine was formed in situ. A carboxylic acid group was then introduced at the C-2 position of the indole in multiple steps, and an amide coupling with 1-adamantylamine gave a tricyclic indole-2-carboxamide. Different derivatives of this product were obtained by alkylations at the indole nitrogen.
The team investigated the correlation between the effects on cannabinoid receptors and the antitumor properties of the synthesized compounds. They used a pediatric glioma cell line to evaluate the anticancer effects. Gliomas represent one of the most common types of brain tumor in children. The researchers found that some of the compounds are non-selective cannabinoid receptor antagonists. One derivative (pictured) showed moderate antitumor activity against the glioma cells. The work shows that the activation of cannabinoid receptors does not necessarily relate to the antitumor effects in gliomas.
- Synthesis and Antitumour Evaluation of Tricyclic Indole‐2‐Carboxamides against Paediatric Brain Cancer Cells,
Alexander John Hamilton, Samuel Lane, Eryn L. Werry, Amreena Suri, Anders W. Bailey, Clémentine Mercé, Ulrich Kadolsky, Alan D. Payne, Michael Kassiou, Simone Treiger Sredni, Alka Saxena, Hendra Gunosewoyo,
ChemMedChem 2024.
https://doi.org/10.1002/cmdc.202400098