The trichloromethyl group is commonly found in bioactive compounds and can also serve as a synthetic intermediate that can be converted to other functional groups such as esters and carboxylic acids. Thus, the development of new trichloromethylation reactions can be useful in both medicinal and synthetic chemistry. However, methods for introducing trichloromethyl groups often require harsh conditions and/or have narrow substrate scopes.
Shinji Harada, Chiba University, Japan, and colleagues have developed a photocatalytic method for the trichloromethylative lactonization of olefins at room temperature (pictured below). The team used a range of compounds with an olefin unit and a carboxylic acid group as substrates and reacted them with BrCCl3 in the presence of fac-Ir(ppy)3 (ppy = 2-phenylpyridine) as a photocatalyst and TsOH•H2O as an acid, using a mixture of dimethylformamide (DMF) and water as the solvent. The reactions were performed at room temperature under blue LED light.
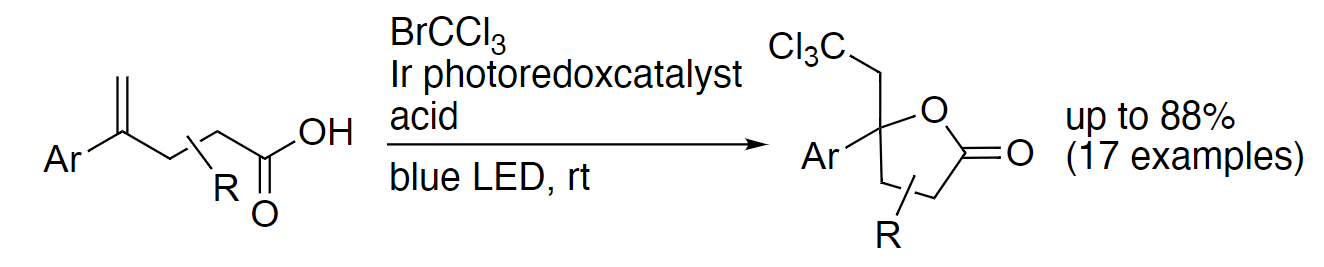
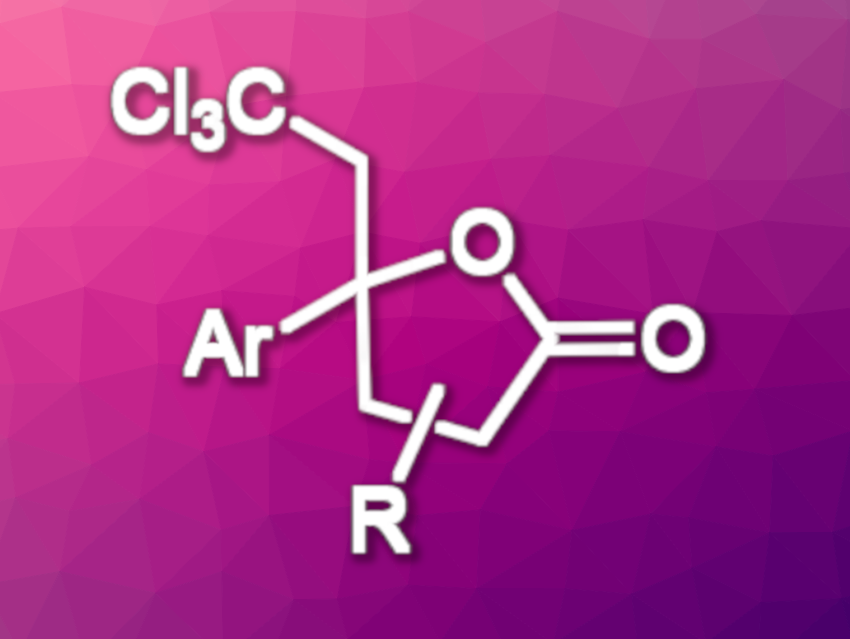
Using this approach, the team obtained a variety of lactones with a tetrasubstituted carbon and a trichloromethyl group in moderate to good yields. The transformation proceeds regio- and stereoselectively. In the reaction, the Br–C bond of bromotrichloromethane is broken to give a trichloromethyl radical (•CCl3) with the help of the activated iridium photocatalyst under blue LED irradiation. The trichloromethyl unit of the products can be further transformed, e.g., into dichloroalkenes, chloroalkynes, or carboxyl groups, leading to useful synthetic building blocks.
- Trichloromethylative Olefin Lactonization by Photoredox Catalysis,
Shinji Harada, Ryotaro Koyama, Ryuya Masuda, Shigeru Arai,
Eur. J. Org. Chem. 2023.
https://doi.org/10.1002/ejoc.202300747