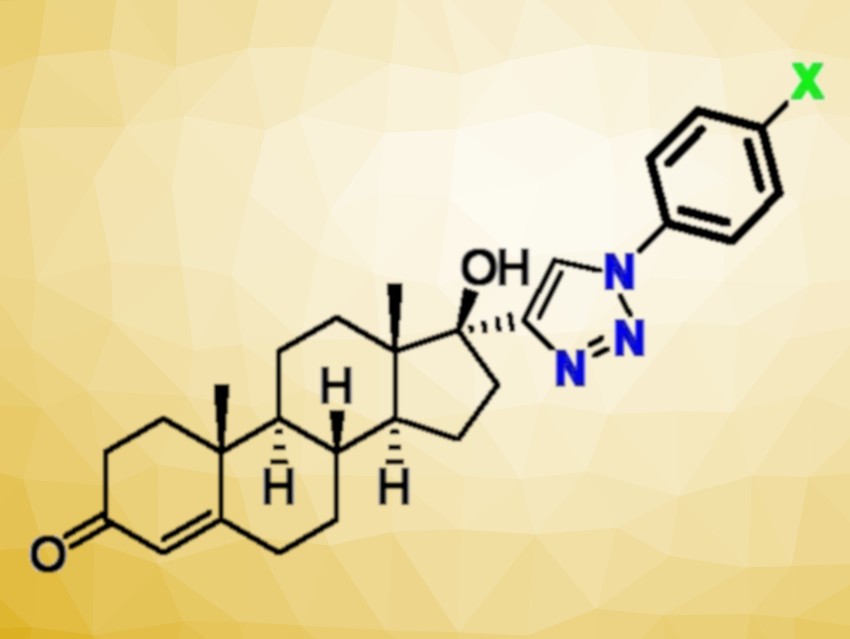
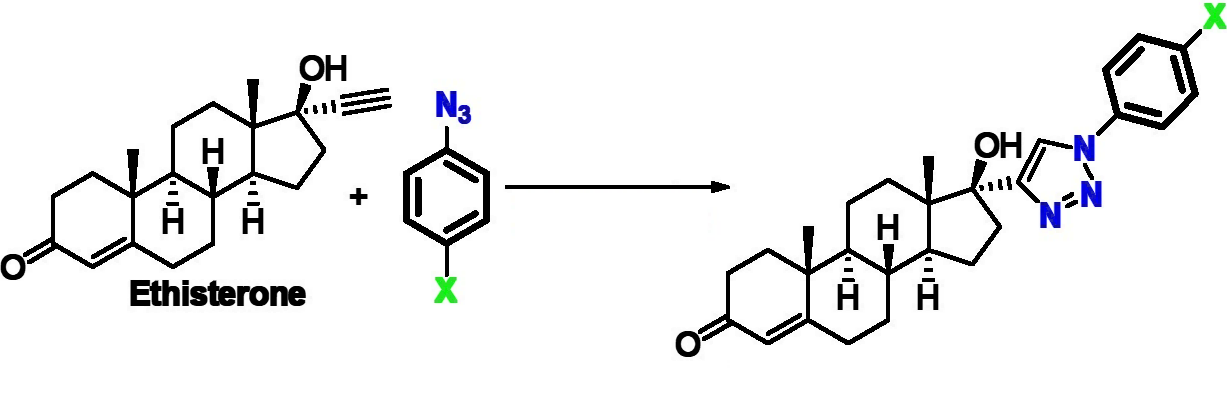
Cancer is a major threat to human health, and the development of new anticancer drugs is an important research target. Ethisterone (pictured below) can be seen as the ethynylated version of testosterone, and ethisterone derivatives have been used as anticancer agents. The ethynyl group can be used for the synthesis of new derivatives, e.g., via the formation of triazoles, which can be useful to develop improved anticancer drug candidates.
Daniel Canseco-González, Universidad Autónoma Chapingo, Texcoco de Mora, México, Alejandro Dorazco-González, David Morales-Morales, Universidad Nacional Autónoma de México, Ciudad de México, and colleagues have developed a series of new D-ring-substituted ethisterones with 1,4-1,2,3-triazoles that can be synthesized in a modular manner. The desired compounds were obtained rapidly in a single synthetic step via an atom-economical click chemistry protocol. Ethisterone was used as a low-cost, commercially available steroid source and was linked to different para-substituted phenyl azides (general transformation pictured below).
The cytotoxic activity of the synthesized derivatives was tested against different human cancer cell lines, including glioblastoma (an aggressive brain cancer), prostate cancer, colorectal cancer, breast cancer, chronic myelogenous leukemia (blood/bone marrow disease), and lung cancer cell lines. Two derivatives with X = Cl and X = I, in particular, showed promising cytotoxicity activities against the leukemia and lung cancer cell lines. The work could be expanded using different steroidal sources to obtain other potentially potent anticancer compounds.
- Facile, Single‐Step Synthesis of a Series of D‐Ring Ethisterones Substituted with 1,4‐1,2,3‐Triazoles: Preliminary Evaluation of Cytotoxic Activities,
Daniel Canseco‐González, Isaac Rodríguez‐Victoria, Teresa Apan‐Ramírez, Alejandro O. Viviano‐Posadas, Juan S. Serrano‐García, Antonino Arenaza‐Corona, Adrian L. Orjuela, Jorge Alí‐Torres, Alejandro Dorazco‐González, David Morales‐Morales,
ChemMedChem 2023.
https://doi.org/10.1002/cmdc.202200659