Alkenes, due to their abundance and versatility, are often used in synthetic applications. Ozonolysis is a well-known transformation that cleaves C=C bonds in alkenes to form carbon-oxygen compounds. However, the analogous aza variant—the cleavage of alkenes to form carbon-nitrogen bonds—has remained largely unexplored.
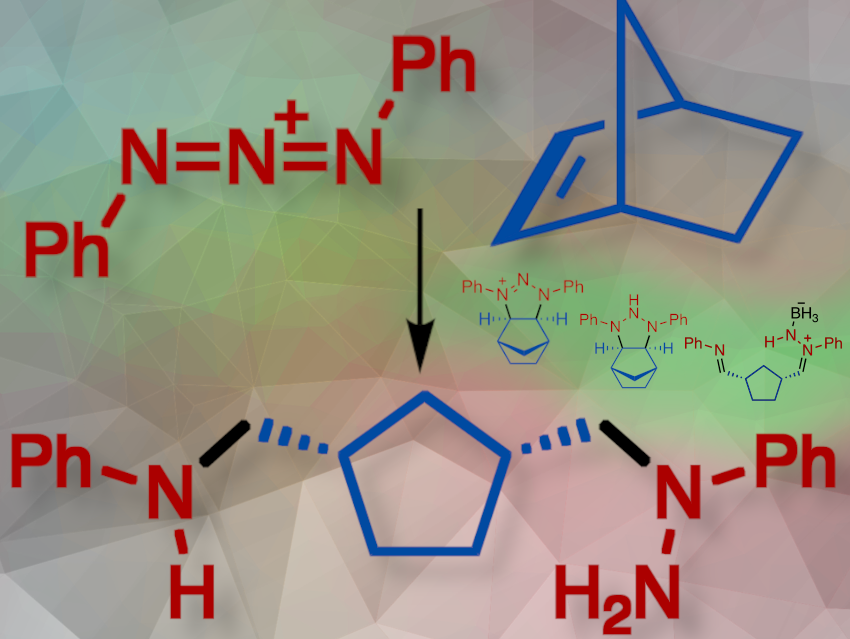
Mark Gandelman and colleagues, Technion – Israel Institute of Technology, Technion City, Israel, have developed ta process called “triazenolysis,” which converts alkenes to amines by fully cleaving the C=C bond. In this reaction, a [3+2] cycloaddition between an alkene and a reactive triazadienium species froms a nitrenium salt (pictured above). This nitrenium salt is then reduced by sodium borohydride (NaBH4) to form an intermediate, N–H triazane. The triazane reacts with a borane-THF complex, producing an adduct, which undergoes reverse cycloaddition to form a BH3-stabilized intermediate. Further reduction with NaBH4 yields the final product. Borane acts as a Lewis acid, enhancing reaction speed and selectivity.
This method is effective for converting cyclic alkenes into diamines and can cleave acyclic C=C bonds to generate two amine moieties. While the rearrangement primarily suits cyclic olefins with C–C bond strain, The researchers found that 1,1-diphenylethylene shows potential for applying triazenolysis to inactivated non-cyclic alkenes. Additionally, triazenolysis of cyclobutenes leads to a novel two-nitrogen atom ring extension.
The researchers say that further development of triazenolysis is currently under investigation in our labs.
- Triazenolysis of alkenes as an aza version of ozonolysis,
Aleksandr Koronatov, Pavel Sakharov, Deepak Ranolia, Alexander Kaushansky, Natalia Fridman, Mark Gandelman,
Nature Chemistry 2024.
https://doi.org/10.1038/s41557-024-01653-3