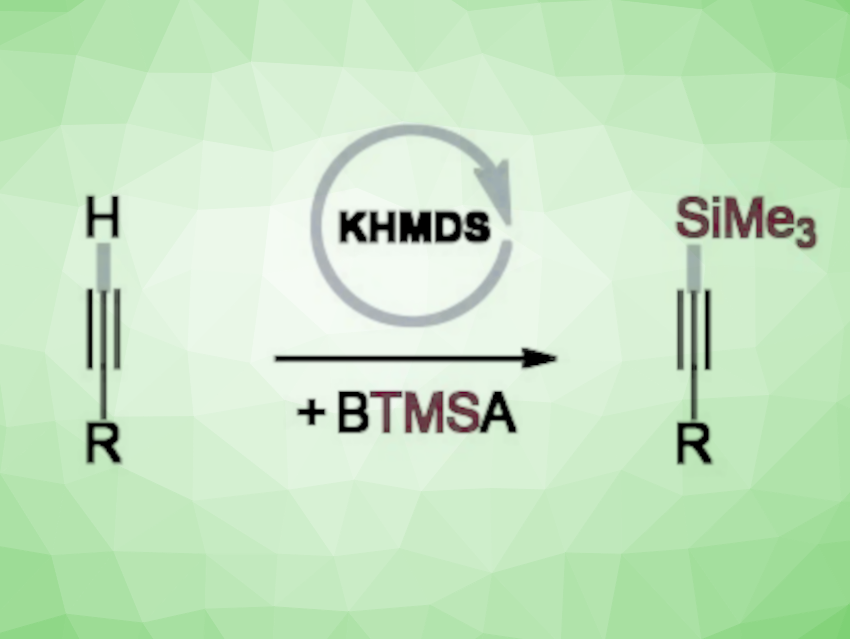
Silylacetylenes, or alkynylsilanes, are useful intermediates in synthesis. They can be readily transformed further, e.g., via cross-coupling reactions, Diels-Alder cyclizations, and cycloadditions. Most known catalytic methods for the synthesis of terminally silylated alkynes are based on transition-metal catalysis.
Krzysztof Kuciński and Grzegorz Hreczycho, Adam Mickiewicz University, Poznań, Poland, have developed an efficient transition metal-free catalytic system for the C–H silylation of terminal alkynes (pictured). The team used bis(trimethylsilyl)acetylene (BTMSA) as the reaction partner, potassium bis(trimethylsilyl)amide (KHMDS) as a commercially available and inexpensive catalyst, and acetonitrile as the solvent. The reactions were performed at room temperature under an argon atmosphere.
The reaction gives a range of trimethylsilylated acetylenes in good to excellent yields of up to 96 %. The late-stage derivatization of commercial pharmaceuticals and the bis-silylation of diynes further demonstrate the potential of the transformation.
The team proposes a reaction mechanism that involves a deprotonation of the terminal alkyne by KHDMS to give an acetylide that reacts with BTMSA. According to the researchers, the approach could be an environmentally benign and sustainable alternative to existing synthetic pathways.
- Transition metal‐free catalytic C−H silylation of terminal alkynes with bis(trimethylsilyl)acetylene initiated by KHMDS,
Krzysztof Kuciński, Grzegorz Hreczycho,
ChemCatChem 2022.
https://doi.org/10.1002/cctc.202200794