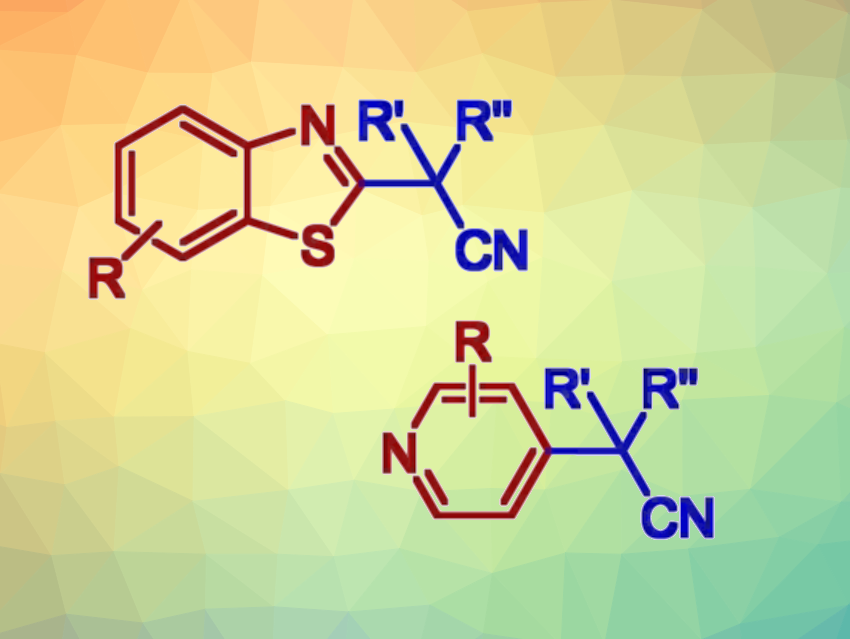
Many small-molecule pharmaceuticals contain nitrogen heterocycles. Thiazoles and pyridines, for example, are privileged scaffolds often used in drug discovery. Thus, methods for the selective C–H functionalization of these heterocycles that enable the late-stage diversification of their structures are in demand.
Ivan Vilotijevic, University of Jena, Germany, and colleagues have developed an efficient method for the selective two-step C–H functionalization of benzothiazoles and pyridines that gives alkylated products (general product structures pictured above). Such transformations have generally required transition-metal catalysts or photocatalysts so far. The team’s transition-metal-free approach is based on selective transformations of benzothiazoles and pyridines to the corresponding heteroarylphosphonium salts (pictured below). This step is followed by reactions of the phosphonium salts with nitrile anions.
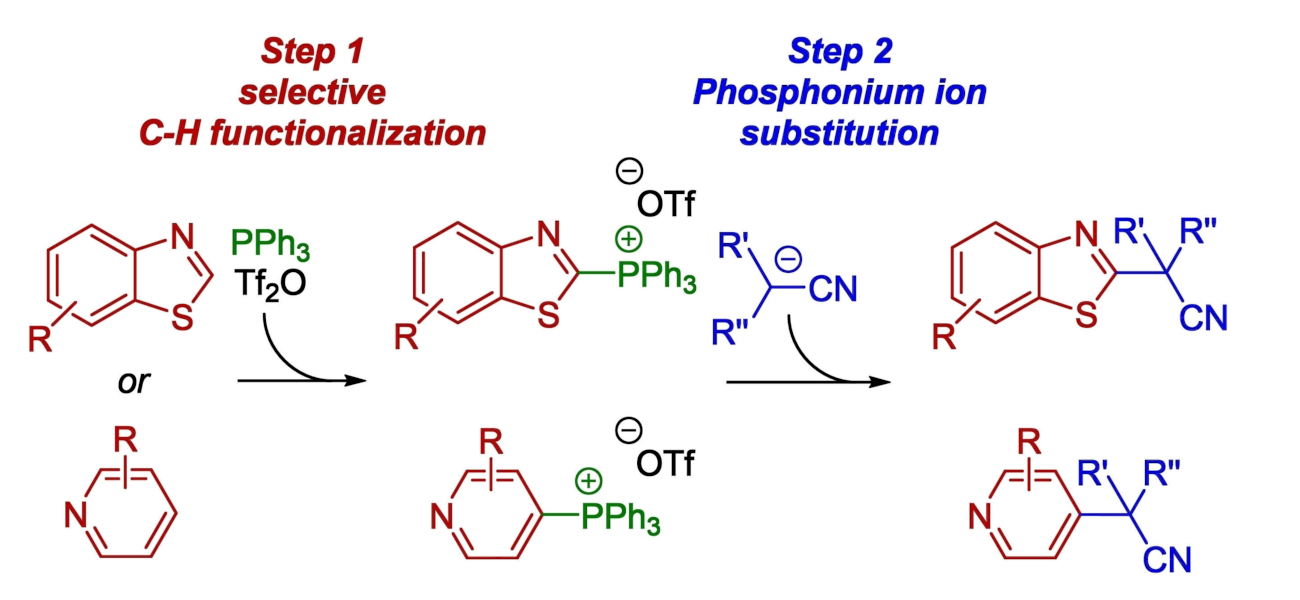
The reactions feature a broad scope and allow the construction of quaternary benzylic carbon centers with high steric hindrance. The site-selectivity of the alkylations is guided by the selectivity of the phosphonium salt formation. The team proposes a nucleophilic aromatic substitution SNAr mechanism for the second step. Overall, the work provides a simple, efficient two-step protocol for the C−H functionalization of benzothiazoles and pyridines under mild conditions.
- Transition‐Metal‐Free Alkylation of N‐Heterocycles with Nitriles via Heteroarylphosphonium Intermediates,
Fritz Schömberg, Milica Perić, Ivan Vilotijevic,
Eur. J. Org. Chem. 2024.
https://doi.org/10.1002/ejoc.202301233