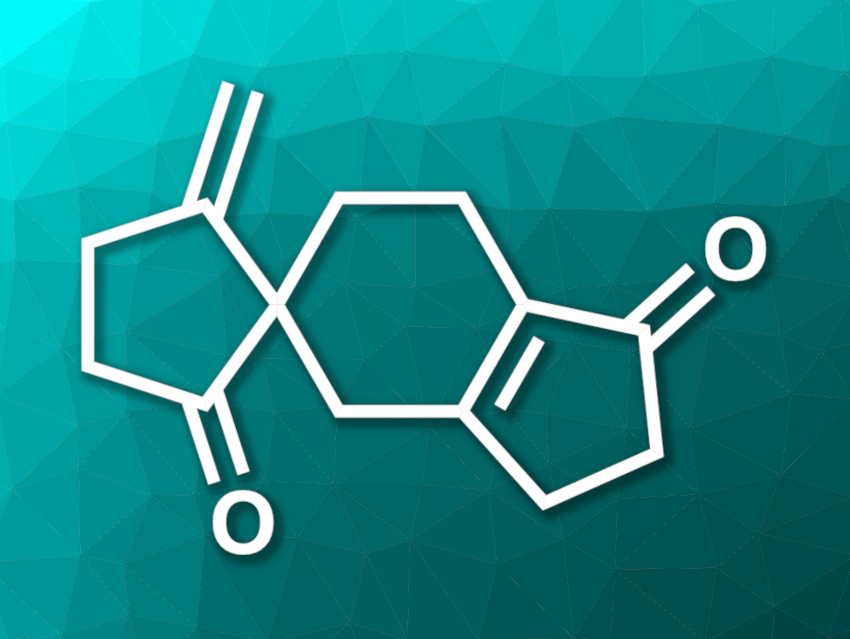
Ulodione A (pictured) is a natural product isolated from the fungus Ulospora bilgramii. The compound has shown some biological activity. It has an unusual 5/6–5 spiro cyclopentanone skeleton and is an interesting target for organic synthesis.
Bor-Cherng Hong, National Chung Cheng University, Chia-Yi, Taiwan, and colleagues have performed the first total synthesis of ulodione A. The team started with a dialkylation of cyclopentane-1,3-dione using 5-bromo-1-nitropentene, which was promoted by N,N-diisopropylethylamine (DIPEA). The resulting spiroalkanedione was then subjected to a semipinacol-like ring-expansion rearrangement, promoted by 1,4-diazabicyclo[2.2.2]octane (DABCO). In this reaction, the spiro[4.4]dione was converted to a spiro[4.5]dione, i.e., a ring expansion from a five-membered to six-membered ring took place.
After these key steps, the resulting nitro-functionalized intermediate was alkylated using an alkyl acrylate, followed by a cyclization to close the second five-membered ring of the target product. This gave a trione, which was converted to the desired product using a selective Wittig olefination.
Overall, the team obtained ulodione A in six reaction steps in a four-pot process. According to the researchers, the new type of semipinacol-like ring-expansion rearrangement used in this synthesis could be a valuable addition to the tools of chemical synthesis.
- Total Synthesis of Ulodione A via a Double-Alkylation and DABCO Promoted Ring-Expansion Rearrangement Sequence,
Chang-Lun Lo, Pavan Sudheer Akula, Bor-Cherng Hong, Gene-Hsiang Lee, Su-Ying Chien,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.2c01038