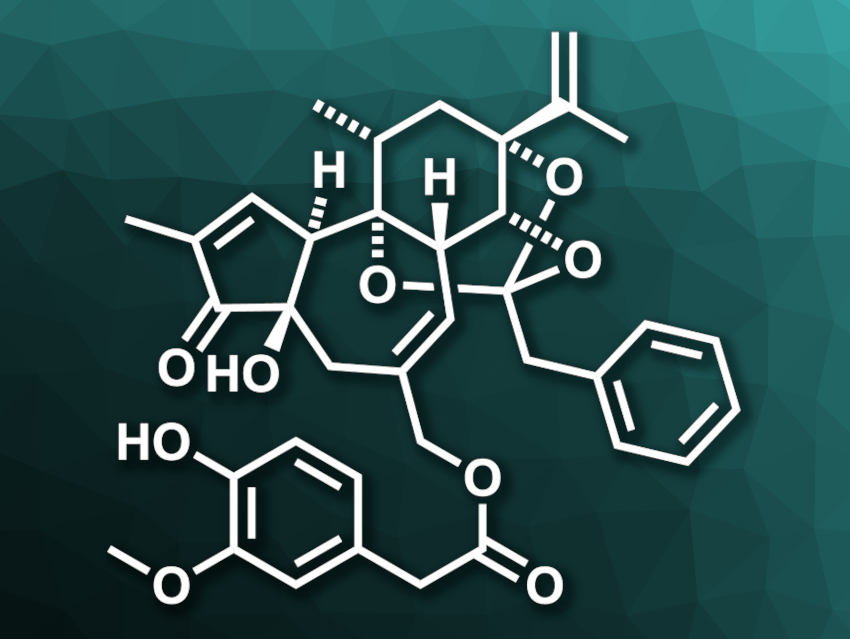
Daphnane diterpene orthoesters (DDOs) are natural products with 5,7,6-fused tricyclic ring systems and embedded orthoesters (three alkoxy groups attached to one carbon atom). They can be bioactive and are found in the plant family Euphorbiacea (spurges). Resiniferatoxin (pictured) is such a bioactive DDO. For example, it is a highly potent capsaicin analogue—pure resiniferatoxin is ca. 500 to 1000 times hotter than pure capsaicin. Due to its complex structure, resiniferatoxin is a challenging target for total synthesis.
Thomas J. Maimone, University of California, Berkeley, USA, and colleagues have developed a 15-step total synthesis of resiniferatoxin. The team started from commercially available (Z)-3-iodopropenoic acid methyl ester, which was reduced to an aldehyde and coupled with the lithium enolate of 3-methylcyclopentenone to introduce a five-membered ring. After further functionalization, the resulting fragment was subjected to a 7-exo Heck cyclization to close the seven-membered ring of the target product.
This was followed by several oxidation-state changes and an aldol coupling with an aldehyde-functionalized fragment that serves as a precursor for the six-membered ring of the 5,7,6-fused tricyclic core. The ring was closed using a radical cyclization with Kagan’s reagent (SmI2). The resulting tricyclic intermediate was converted into the desired resiniferatoxin in five steps. The team aims to extend the developed strategy to the synthesis of other DDOs.
- Total Synthesis of Resiniferatoxin,
Vasil H. Vasilev, Lukas Spessert, Kuan Yu, Thomas J. Maimone,
J. Am. Chem. Soc. 2022.
https://doi.org/10.1021/jacs.2c08200


![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)
