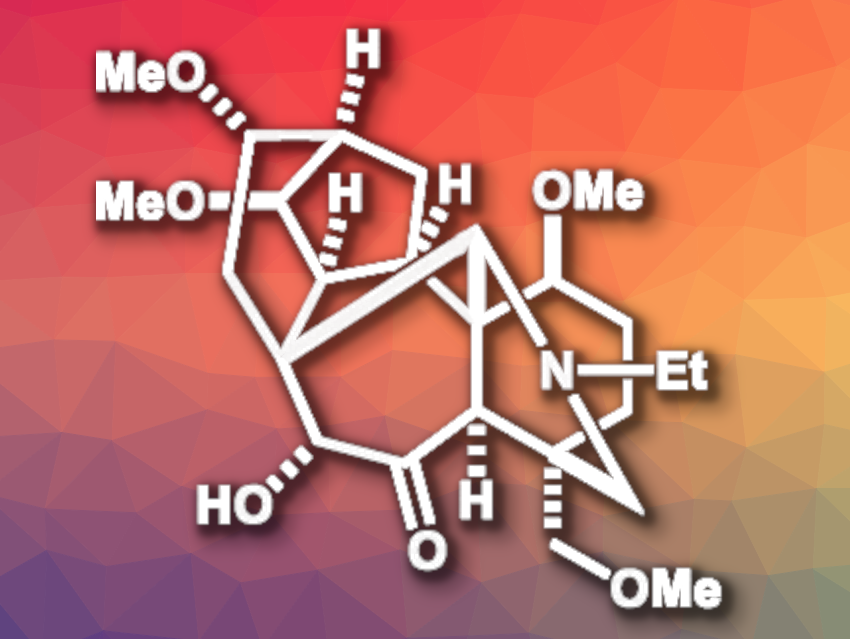
Puberuline C (pictured) is a structurally complex C19-diterpenoid alkaloid with a 6/7/5/6/6/6 ring framework. C19-diterpenoid alkaloids often have interesting biological activities. However, the limited natural supply of puberuline C has so far prevented detailed bioactivity studies. Until now, there had been no reported total synthesis of puberulin C.
Masayuki Inoue, The University of Tokyo, Japan, and colleagues have performed the first total synthesis of puberulin C. The team started from 2-cyclohexenone, which forms the A ring of the target compound. A double Mannich reaction was then used to introduce the E ring. After several functionalization steps, the AE ring fragment was combined with lithium TMS-acetylide, allyl bromide, and 2-iodocyclopentenone as the C ring fragment.
The resulting intermediate undergoes a radical cascade reaction that closes both the B and F rings of puberuline C and installs five contiguous stereocenters in the process. An intramolecular Mukaiyama aldol reaction was used to form the D ring. Puberuline C was then obtained via further functionalization reactions. The target compound was prepared in 32 total steps from 2-cyclohexenone. According to the researchers, the work could also be useful for the synthesis of other C18– and C19-diterpenoid alkaloids.
- Total Synthesis of Puberuline C,
Tsukasa Shimakawa, Shu Nakamura, Hibiki Asai, Koichi Hagiwara, Masayuki Inoue,
J. Am. Chem. Soc. 2022.
https://doi.org/10.1021/jacs.2c11259