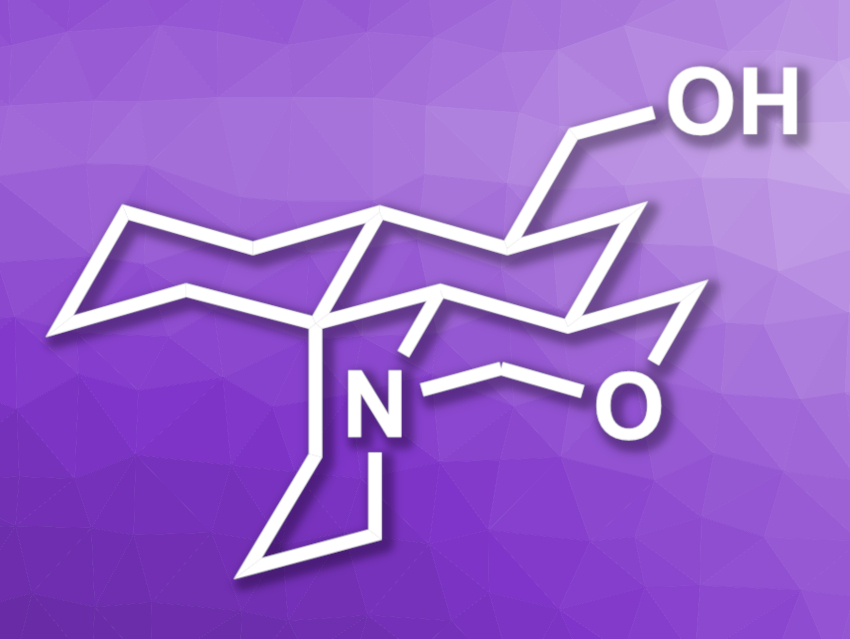
Myrioneurinol (pictured) is part of a family of alkaloids made up of polycyclic natural products isolated from Myrioneuron plants. The compounds in this family show interesting bioactivities, e.g., antimalarial, antiviral, and cytotoxic activity. The structure of myrioneurinol features fused piperidine, oxazine, and cyclohexane rings and five stereogenic centers, making it an interesting target for total synthesis.
Jake M. Aquilina and Myles W. Smith, UT Southwestern Medical Center, Dallas, TX, USA, have performed a total synthesis of myrioneurinol, including the first asymmetric approach to the compound via a formal synthesis. The team used a desymmetrization approach from a symmetrical precursor to assemble the core of the product stereoselectively.
The researchers started from pentachlorocyclopropane, which was converted to a bicyclic chlorodiketone and further to a bicyclic diketo aldehyde. In a key step, this intermediate was subjected to a desymmetrizing double reductive amination to form the piperidine unit. When an enantiopure chiral amine is used in this step, the approach allows for an asymmetric formal synthesis of myrioneurinol.
Overall, the team synthesized myrioneurinol in 18 steps and a yield of about 1 %. According to the researchers, the symmetry-driven approach could also be useful for other alkaloid targets.
- Symmetry-Driven Total Synthesis of Myrioneurinol,
Jake M. Aquilina, Myles W. Smith,
J. Am. Chem. Soc. 2022.
https://doi.org/10.1021/jacs.2c04487