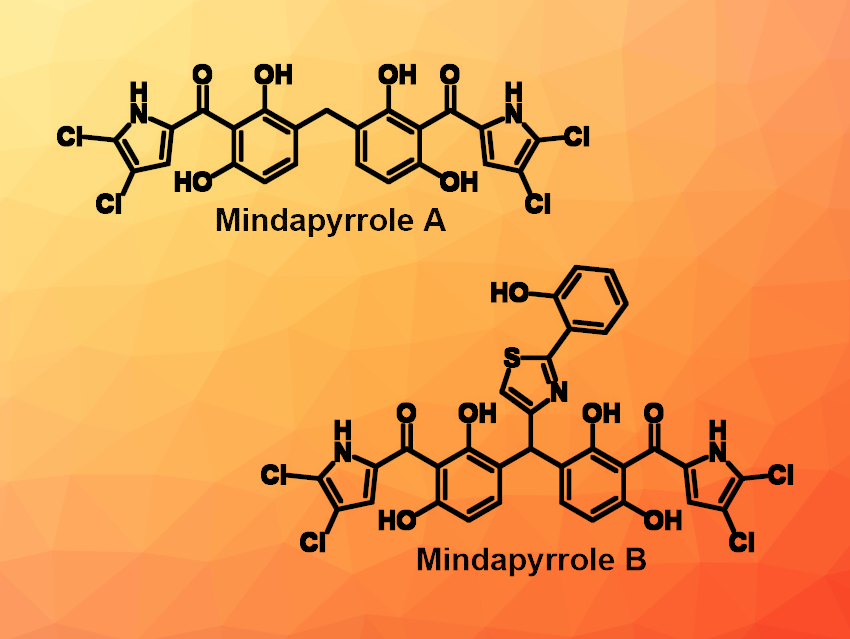
Antibiotic resistance is an ever-evolving problem that requires continued drug development to keep pace with pathogenic microbes. One way of approaching this threat is the investigation of natural products that have novel or multiple mechanisms of action. In this context, Ryan A. Allen and William M. Wuest, Emory University, Atlanta, GA, USA, have performed a total synthesis and biological investigation of mindapyrroles A and B (pictured), two recently isolated natural products whose mechanisms of action had been unknown so far.
The team started with the synthesis of pyoluteorin, which is the monomeric unit of the mindapyrroles, via a Friedel-Crafts acylation of pyrrole with 2,6-dimethoxybenzoic acid, followed by deprotection/protection steps and dichlorination. The key step in the synthesis of mindapyrrole A then involved using dimethoxymethane in a Friedel–Crafts dimerization of pyoluteorin. In a similar manner, mindapyrrole B was synthesized using aeruginaldehyde as a coupling partner for pyoluteorin. This work represents the second total synthesis of mindapyrrole A and the first synthesis of mindapyrrole B.
The products were then tested for their antimicrobial activity against a panel of gram-positive and gram-negative bacterial pathogens. The team found that they are most active against gram-positive pathogens. They also discovered that the mindapyrroles likely inhibit bacterial growth via a different mechanism than the monomer pyoluteorin. Further investigation will be necessary to identify the alternative mechanisms of action of the mindapyrroles.
- Total synthesis and biological investigation of mindapyrroles A and B,
Ryan A. Allen, William M. Wuest,
ChemMedChem 2023.
https://doi.org/10.1002/cmdc.202300235