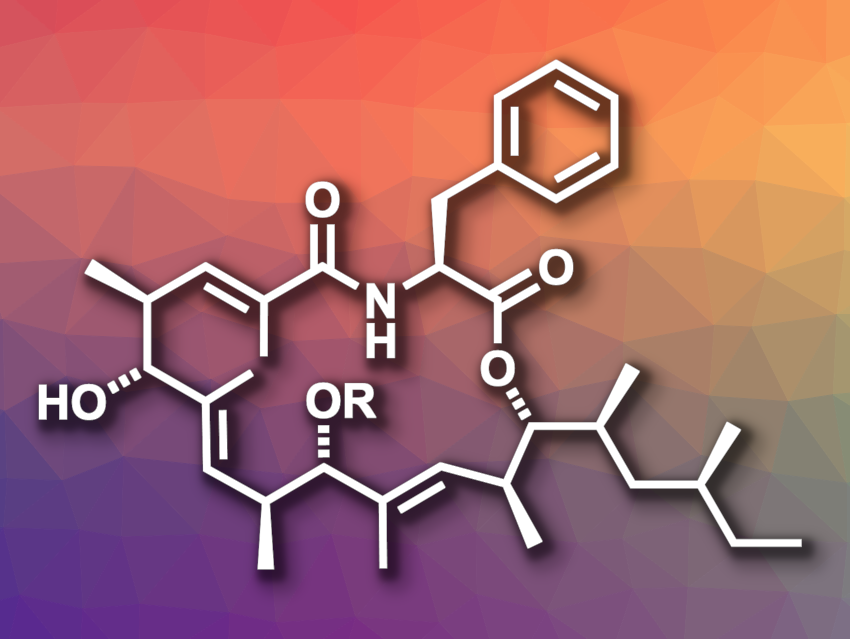
Metacridamides A and B (pictured; R = Ac and H, respectively) are natural products that were isolated from a pathogenic fungus that infects grasshoppers. The compounds’ structure features a 17-membered macrocycle and their absolute configuration had been determined by X-ray single crystal analysis. They have cytotoxic properties, and further studies of their mechanism of action would be interesting.
Masahito Yoshida, University of Tsukuba, Ibaraki, Japan, and colleagues have performed a concise total synthesis of metacridamides A and B and revised the absolute configuration at the C-9 position of the compounds. The team used repeated vinylogous Mukaiyama aldol reactions (VMARs) and an ynamide-mediated macrolactonization.
They started from (S)-2-methyl-1-butanol, which was converted to an aldehyde building block. This aldehyde was used in the first VMAR together with an N,O-ketene acetal to build a larger, α,β-unsaturated aldehyde. This was followed by two more VMARs with the same N,O-ketene acetal to obtain an allyl ester building block. This intermediate was then converted to a suitable precursor for cyclization by removal of the allyl group, amidation, and protection/deprotection steps. An ynamide-mediated macrolactonization led to the desired macrocycle, which was converted to metacridamide A by deprotection. Metacridamide B was obtained by removing the acetyl group.
The team found that the spectral data of the prepared compounds was identical to those of the natural products. Based on this data, they revised the absolute configuration at the C-9 position of the compounds to S.
- Concise total synthesis and structure revision of metacridamides A and B,
Masahito Yoshida, Yuhi Okoshi, Hideo Kigoshi,
Chem. Commun. 2023.
https://doi.org/10.1039/d3cc01694c