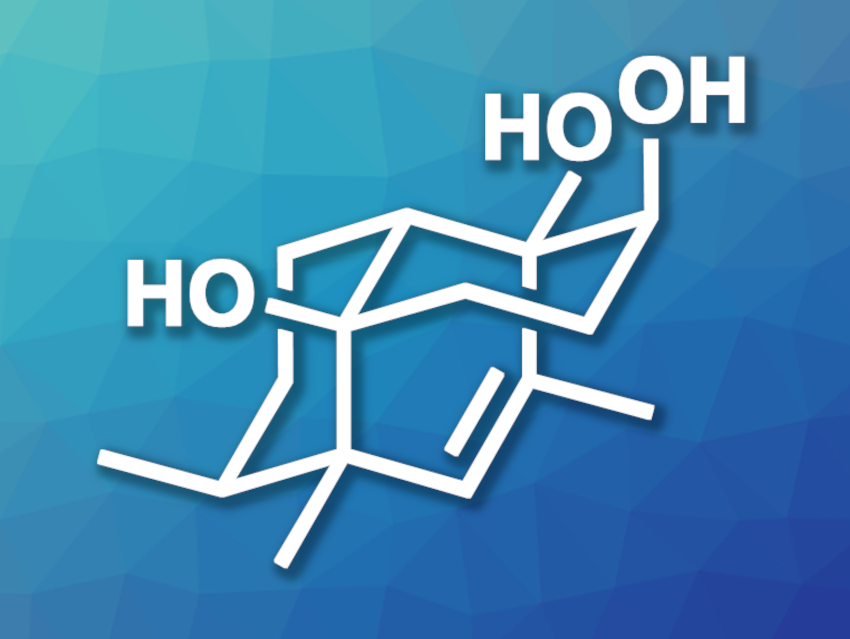
Lemnardosinane A (pictured) is a sesquiterpenoid natural product that was isolated from marine soft corals found in the South China Sea. The compound has a tricyclic 6/6/6 skeleton and has shown interesting biological activities. So far, there had been no synthesis of lemnardosinane A.
Hisanaka Ito, Tokyo University of Pharmacy and Life Sciences, Japan, and colleagues have performed the first asymmetric total synthesis of lemnardosinane A. The team used an intramolecular aldol reaction, the stereoselective introduction of an alkyne group, and an intramolecular pinacol coupling as the key steps. They started from (S)-carvone, which was methylated and allylated. The resulting intermediate underwent a hydroboration-oxidation, the isopropenyl group was removed, and further hydrogenation and oxidation steps were used to obtain an aldehyde intermediate. The aldehyde was used in an intramolecular aldol cyclization to close the second six-membered ring.
The next steps were the protection of the alcohol in the resulting bicyclic intermediate and the stereoselective introduction of a protected propargyl alcohol unit. The resulting alkyne was hydrogenated, followed by an oxidation step and treatment with Tf2O to introduce a vinyl triflate unit. A cross-coupling with methylmagnesium iodide was then used to install a methyl group at the double bond. Further deprotection/protection steps and a Dess–Martin oxidation gave a precursor for the intramolecular pinacol coupling that the team used to close the third ring. Finally, deprotection gave lemnardosinane A. The product was obtained in an overall yield of 0.5 % in 20 steps from (S)-carvone.
- Asymmetric Total Synthesis of (+)-Lemnardosinane A,
Toyoharu Kobayashi, Futaba Hayano, Akinobu Kamiya, Yuichiro Kawamoto, Hisanaka Ito,
Org. Lett. 2024.
https://doi.org/10.1021/acs.orglett.4c01384