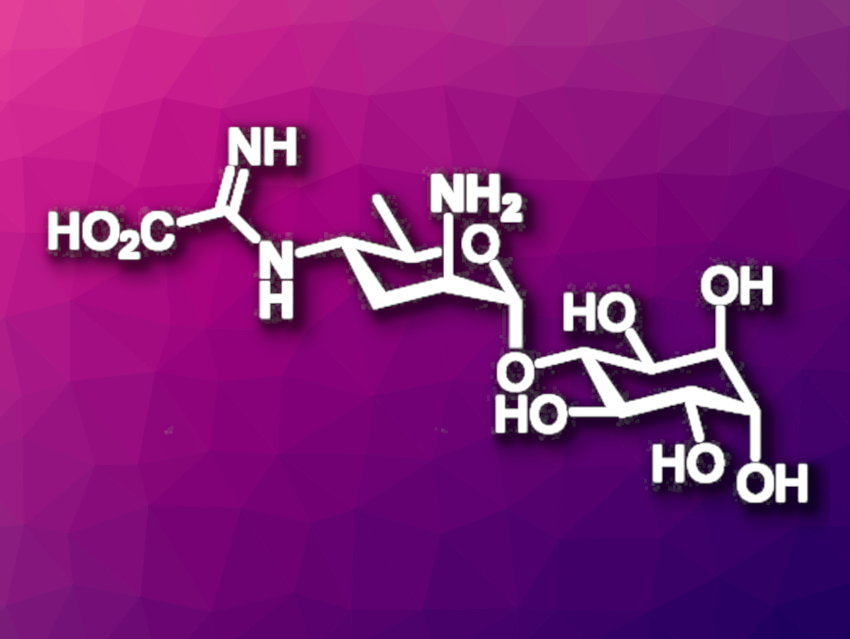
Kasugamycin (pictured) is an aminoglycoside antibiotic. Its structure features an unusual 2,3,4,6-tetradeoxy-2,4-diaminopyranoside unit. Synthetic paths to kasugamycin and its derivatives could allow researchers to develop analogues with useful biological properties. However, its characteristic multideoxy diamino sugar unit, which is called kasugamine, makes kasugamycin a somewhat challenging target for total synthesis.
Qian Wan, Huazhong University of Science and Technology, Wuhan, Hubei, China, Wuhan University, Hubei, China, Jing Zeng, Huazhong University of Science and Technology, and colleagues have developed an efficient synthetic pathway for the preparation of kasugamycin that uses D-fucal as the starting material. This glycal (a cyclic enol ether derivative of a sugar) can be obtained on a large scale from galactose.
The team first removed the allylic acetoxy group of D-fucal via a reductive cleavage to give a 3-deoxyglycal, followed by deacetylation. The resulting glycal intermediate was converted to an azido-substituted compound in two steps and further transformed to a nosyl (4-nitrobenzenesulfonamide) derivative. Then a tandem epoxidation-glycosylation was used to obtain a 2-hydroxy glycosyl m-chlorobenzoate, and the C-2 amino group was introduced via another azide intermediate. A glycosylation reaction with a D-inositol derivative and the introduction of the amidine unit then led to the desired kasugamycin in the form of its hydrochloride.
According to the researchers, the developed approach could also allow for the synthesis of kasugamine derivatives. It may, thus, be helpful for the development of new analogues with useful biological activities.
- Total Synthesis of Kasugamycin,
Ting Li, Yaling Xiang, Lingkui Meng, Qian Wan, Jing Zeng,
Org. Lett. 2025.
https://doi.org/10.1021/acs.orglett.4c04545