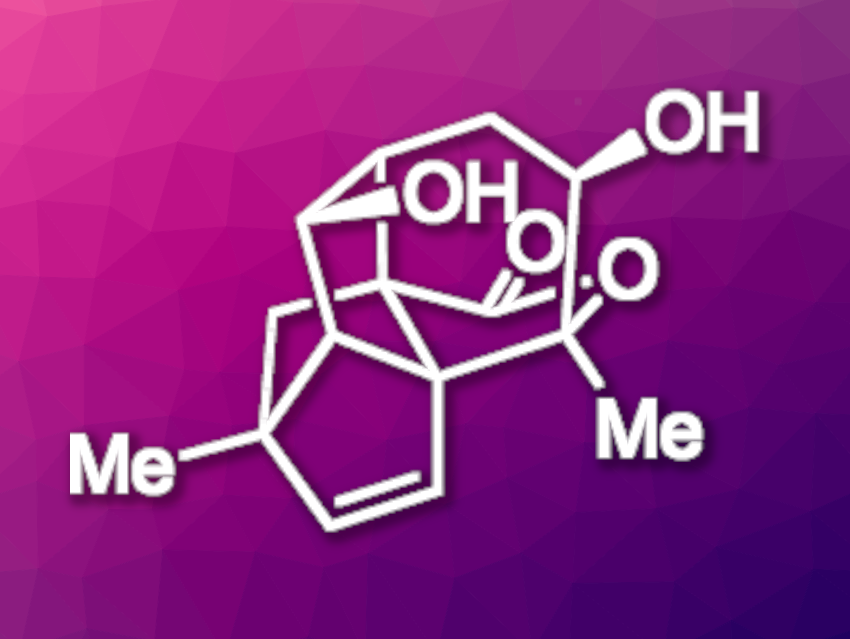
Artemisia plants contain many interesting, bioactive natural products, such as the antimalaria drug artemisinin. Artatrovirenol A (pictured) is a natural product that was insolated from Artemisia atrovirens. It has shown cytotoxic activity against liver cancer cell lines. The compound has a complex, cage-like structure with a 5/5/6/5/5-pentacyclic system featuring a norbornene substructure. It is an interesting target for total synthesis.
Jieping Zhu, Ecole Polytechnique Fédérale de Lausanne (EPFL), Switzerland, and colleagues have performed an enantioselective total synthesis of (−)-artatrovirenol A. The team started by preparing a bycyclic silyl ketene acetal, which was reacted with a known cyclic enone in a Mukaiyama–Michael addition to obtain an intermediate that contains all 15 carbon atoms of the desired product.
An intramolecular de Mayo [2 + 2] cycloaddition/retro-aldol sequence was then used to build the tetracyclic one of the target compound. The ring opening of an epoxide was used to create a norbornane substructure, which was then converted to the corresponding norbornene using a Chugaev elimination. The team obtained ca. 100 mg of artatrovirenol A in one synthesis run.
- Enantioselective Total Synthesis of (−)-Artatrovirenol A,
Rémi Lavernhe, Patrick Domke, Qian Wang, Jieping Zhu,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c09683