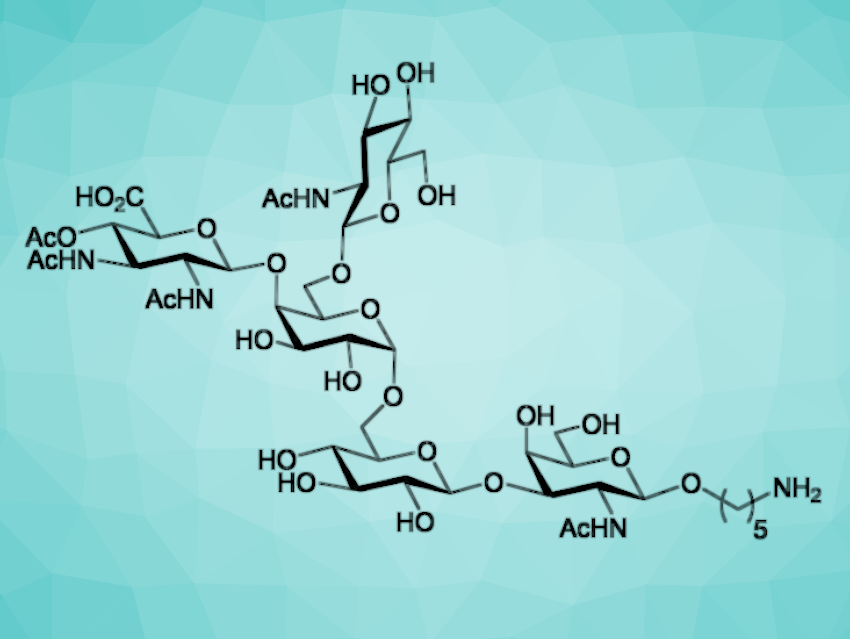
The chemical synthesis of bacterial glycans has typically focused on the production of repeating units of capsular polysaccharides. Such species have proven useful for the development of glycoconjugate vaccines. Less attention has been paid to the synthesis of bacterial O-glycans, which are attached to surface glycoproteins by O-glycosidic bonds.
Dancan K. Njeri and Justin R. Ragains, Louisiana State University, Baton Rouge, USA, have synthesized a conjugation-ready analog of a pentasaccharide O-glycan (pictured) associated with the drug-resistant Gram-negative bacterial species Acinetobacter baumannii. Previous studies suggest that this O-glycan is present on the surface of a variety of strains and that it is important for their fitness as pathogens. This O-glycan could also play an important role in the development of glycoconjugate vaccines.
The researchers first generated an unusual 2,3-diacetamido-4-acetyl-2,3-dideoxy-D-glucuronic acid (DADA) unit, starting from known 2-deoxy-2-trichloroacetamido-D-glucose-1,2,4,6-tetraacetate. As a second building block, they prepared an N-acetyl-D-galactosamine (GalNAc) derivative from a known thioglycoside. Further fragments were based on D-glucose (Glc), D-galactose (Gal), and N-acetyl-D-glucosamine (GlcNAc), and a precursor for the amine linker. The fragments were then assembled stepwise to obtain the desired pentasaccharide, functionalized with the linker.
The team’s synthesis involves a longest linear sequence of 19 steps. Challenges addressed in the synthesis included the development of an effective protecting group scheme and the late-stage installation of a synthetically challenging glycosidic linkage. This work could be useful for studies on the chemical immunology of A. baumannii.
- Total Synthesis of a Pentasaccharide O‐Glycan from Acinetobacter baumannii,
Dancan K. Njeri, Justin R. Ragains,
Eur. J. Org. Chem. 2022.
https://doi.org/10.1002/ejoc.202201261