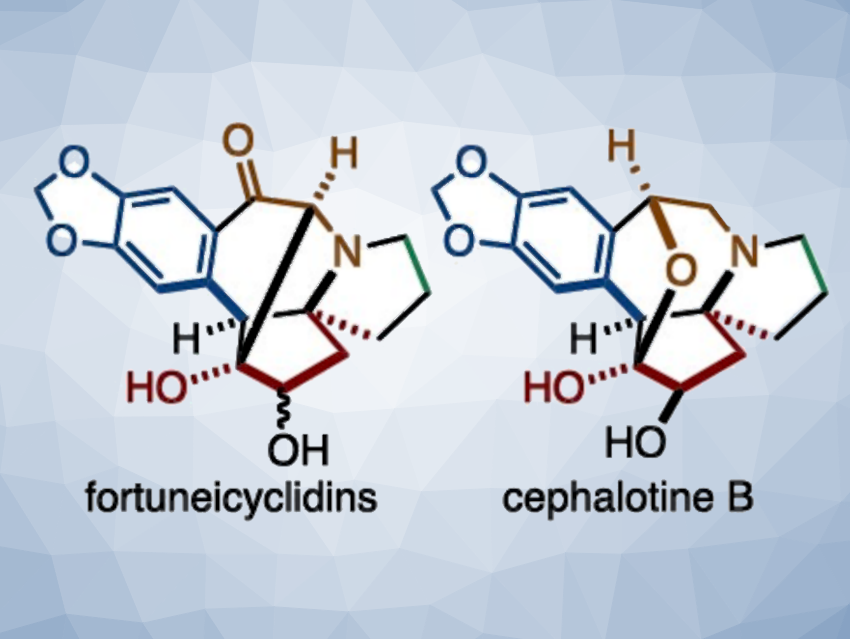
The Cephalotaxus alkaloids are natural products isolated from the plant Cephalotaxus, a type of conifer. The family of these natural products includes, e.g., an FDA-approved anti-leukemia drug. The alkaloids have a unique pentacyclic system consisting of a tetrahydrobenzazepine and an azaspirocycle. Among them, fortuneicyclidins and cephalotines have fairly complex structures, which are interesting and challenging targets for total synthesis.
Junichiro Yamaguchi, Waseda University, Tokyo, Japan, and colleagues have developed a concise synthesis of fortuneicyclidins A and B, and cephalotine B (pictured). One key step in the synthesis is a Pd-catalyzed dearomative spirocyclization of bromofurans with N-tosylhydrazones. A subsequent tandem reaction involving a skeletal rearrangement and Friedel–Crafts cyclization was used to construct the main pentacyclic skeleton. After that, two metal-catalyzed hydrosilylations were used for chemoselective carbonyl transformations, minimizing protecting group manipulations.
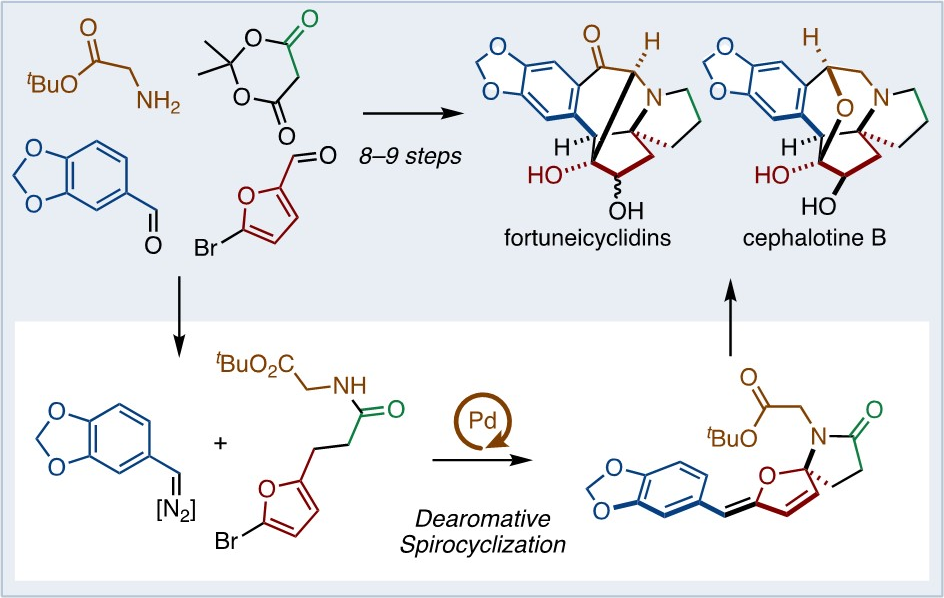
The team accomplished the total syntheses of fortuneicyclidins A and B in eight steps and that of cephalotine B in nine steps. According to the researchers, the work demonstrates that the appropriate
use of tandem reactions can enable the rapid synthesis of complex molecular skeletons.
- Concise Synthesis of (±)‐Fortuneicyclidins and (±)‐Cephalotine B Enabled by Pd‐Catalyzed Dearomative Spirocyclization,
Yota Uwabe, Kei Muto, Junichiro Yamaguchi,
Chem. Eur. J. 2023.
https://doi.org/10.1002/chem.202302769