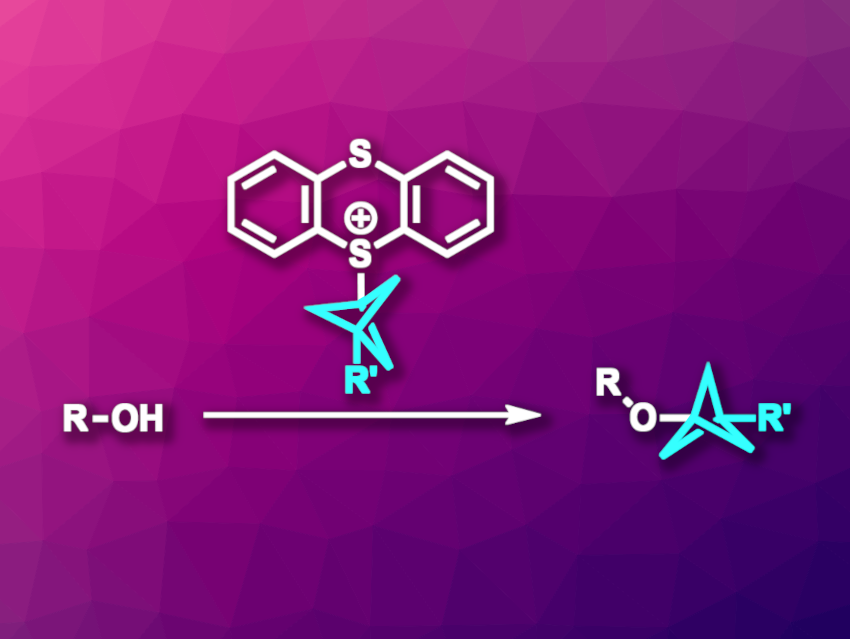
In pharmaceutical chemistry, 1,3-disubstituted bicyclo[1.1.1]pentyl (BCP) units can serve as alternatives to para-substituted arene units. This is useful for tuning the properties of drug candidates without changing their structure too much. Thus, methods for the preparation of BCP derivatives are useful. While there are approaches to the synthesis of, e.g., BCP amines, arenes, and alkanes, so far, the synthesis of BCP alkyl ethers from alcohols had not been achieved.
Tobias Ritter, Max Planck Institute for Coal Research, Mülheim an der Ruhr, Germany, and colleagues have developed the first method for the synthesis of BCP alkyl ethers from alcohols (pictured). The team used BCP–thianthrenium (TT+) reagents for this type of bicyclopentylation reaction, together with an iridium-based photoredox catalyst and a copper catalyst. They reacted a variety of primary, secondary (both cyclic and acyclic), and tertiary alcohols with e.g., a trifluoromethyl bicyclopentyl thianthrenium salt or an analogous cyano-substituted reagent. The team used either Cu(acac)2 (acaca = acetylacetonate) or Cu(TMHD)2 (TMHD = 2,2,6,6-tetramethyl-3,5-heptanedionate) as the copper catalyst, Ir[dF(CF3)ppy]2(dtbbpy)PF6 as the photocatalyst, Na2CO3 as a base, and MeCN as the solvent. The reactions were performed in the presence of 3 Å molecular sieves under blue LED light at 30 °C.
The desired products were obtained in yields of 61–90 %. The approach tolerates various synthetically useful functional groups. According to the researchers, the method proceeds via a metal-mediated radical process.
- Bicyclopentylation of Alcohols with Thianthrenium Reagents,
Zibo Bai, Beatrice Lansbergen, Tobias Ritter,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c10024


![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)
