Most enzyme biocatalysts, including monooxygenases and peroxygenases, have low thermostability, which hampers their use. Thermostable protein folds of natural and synthetic origin are, thus, interesting templates for biocatalyst development due to their enhanced stability under elevated temperatures. Cytochrome P450 enzymes (CYPs) are a family of heme-thiolate monooxygenases that catalyze the oxidation of their substrates in a highly stereo- and regio-selective manner. The ability to engineer the existing stable folds of thermostable CYPs into efficient peroxygenases, whilst maintaining their high levels of selectivity, would be useful.
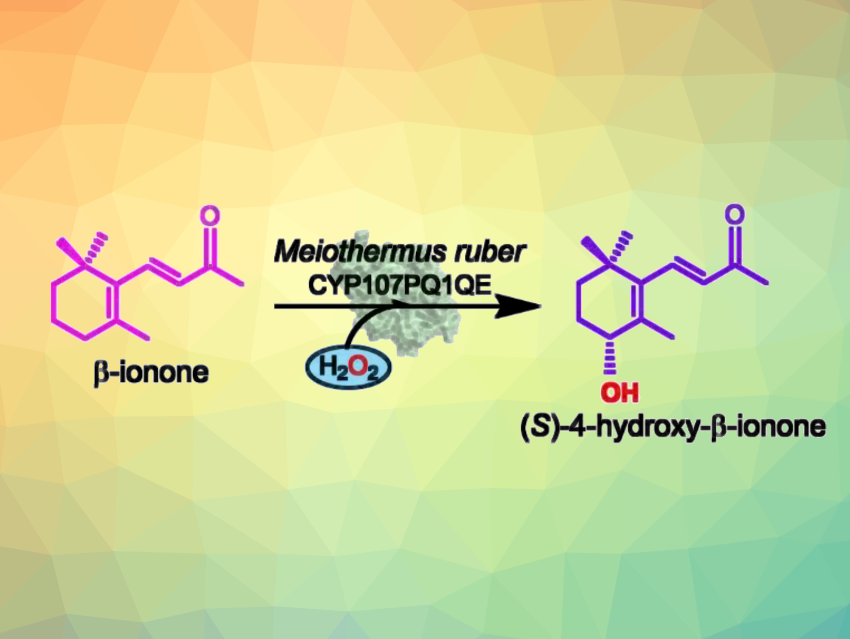
Stephen Graham Bell, The University of Adelaide, Australia, and colleagues have used a CYP enzyme (CYP107PQ1) from the thermophilic bacterium Meiothermus ruber that binds the norisoprenoid β-ionone as a template for catalyst design. The team modified the enzyme’s I-helix to convert the enzyme from a monooxygenase into a peroxygenase (CYP107PQ1QE). The engineered enzyme enabled the enantioselective oxidation of β-ionone to (S)-4-hydroxy-β-ionone (pictured) with high enantioselectivity (94 % e.e.).
The enzyme was resistant, e.g., to the H2O2 solution used as the oxidant or the presence of an organic solvent. It supported over 1,700 turnovers and was fully functional after incubation at 60 °C for 1 h or at 30 °C for 365 days. The reaction was scaled up to generate multi-milligram quantities of the product for characterization. Overall, the team successfully used a CYP protein fold from a thermophilic bacterium to design a highly stable enzyme for stereoselective C–H bond activation. The work could provide a useful strategy for future biocatalyst design.
- A Thermostable Heme Protein Fold Adapted for Stereoselective C‐H Bond Hydroxylation Using Peroxygenase Activity,
Tuhin Das, Eva F. Hayball, Alix C. Harlington, Stephen Graham Bell,
ChemBioChem 2024.
https://doi.org/10.1002/cbic.202400737