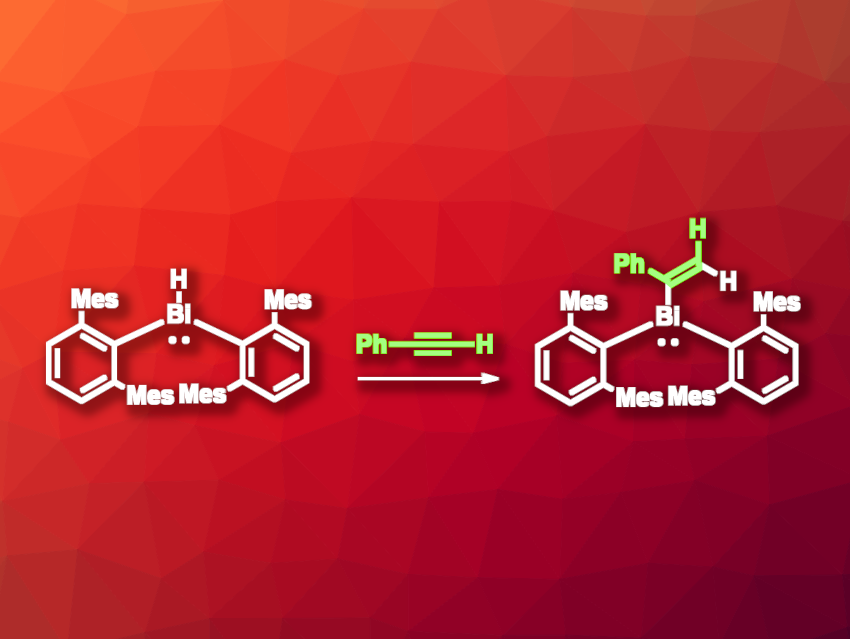
The chemistry of bismuth compounds is interesting, e.g., due to the redox-active nature of the element, which can be useful in different types of reactions. Little is known about the reactivity of bismuth hydrides, which are generally considered to be unstable. The only known thermally stable complex containing a Bi–H bond is [Bi(H)(2,6-Mes2C6H3)2] (Mes = 2,4,6-trimethylphenyl). This compound is stabilized by the bulky mesityl groups.
Using this bismuth hydride, Heikki M. Tuononen, University of Jyväskylä, Finland, Philip P. Power, University of California, Davis, USA, and colleagues have performed the first example of a hydrobismuthation reaction. The team reacted the bismuth hydride with phenylacetylene, giving the Markovnikov addition product [(2,6-Mes2C6H3)2BiC(Ph)═CH2] (pictured). The reaction was performed at 60 °C in toluene, and the product was isolated in a yield of 83 % in the form of an orange powder. Suitable crystals could be grown and the product was characterized using single-crystal X-ray diffraction.
The researchers also tried to activate ethylene with the bismuth hydride under the same conditions. They monitored the reaction using 1H NMR spectroscopy and found evidence for the formation of small amounts of a bismuth–ethyl species. However, the dibismuthene [Bi(2,6-Mes2C6H3)]2 and the terphenyl arene 2,6-Mes2C6H4 were also formed and ethylene was regenerated, showing that ethylene is a poor substrate for the hydrobismuthation. According to the researchers, the hydrobismuthation of other unsaturated organic substrates will be investigated.
- Hydrobismuthation: Insertion of Unsaturated Hydrocarbons into the Heaviest Main Group Element Bond to Hydrogen,
Kristian L. Mears, Gia-Ann Nguyen, Bronson Ruiz, Annika Lehmann, Jonah Nelson, James C. Fettinger, Heikki M. Tuononen, Philip P. Power,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.3c06535