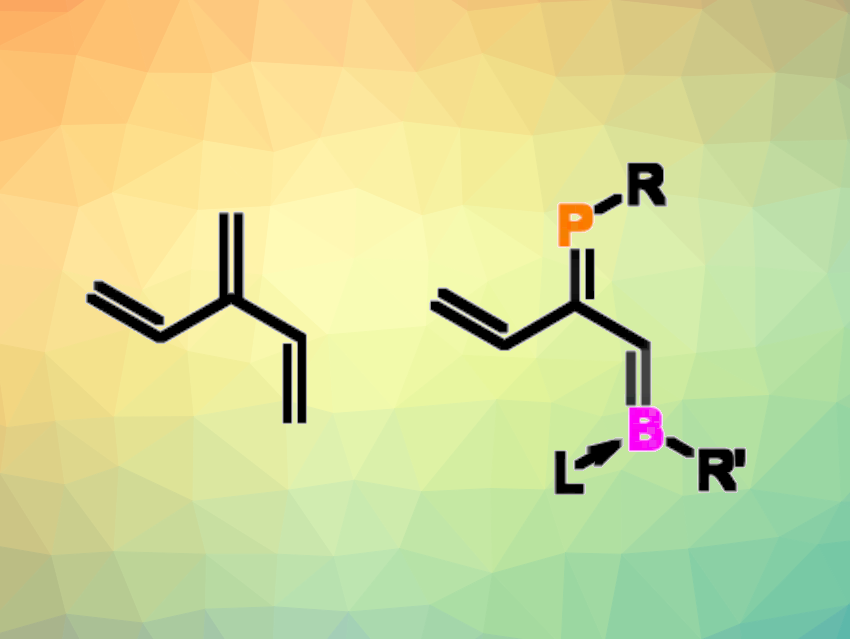
Dendralenes are acyclic cross-conjugated polyenes. The simplest example is buta-1,3-diene, or [2]dendralene, followed by [3]dendralene (pictured above on the left). The properties of such unsaturated compounds can be tuned by incorporating heteroatoms. For example, units with two carbon atoms can replaced by ones with one N and one B atom. However, there had been no examples of [n]dendralenes with heavier main-group elements, such as phosphorus, so far.
Michael J. Cowley, University of Edinburgh, UK, and colleagues have synthesized the first heavy heterodendralenes, formally obtained by isoelectronic replacement of two carbon atoms with a phosphorus/boron pair (simplified general structure pictured above on the right). The team used a ring-opening reaction of 1,2-phosphaboretes—unsaturated four-membered rings with adjacent P and B atoms. The ring opening is base-induced. In the presence of N-heterocyclic carbenes (NHCs) such as 1,3,4,5-tetramethylimidazol-2-ylidene in benzene, the 1,2-phosphaboretes are transformed into phosphabora[3]dendralenes, which the NHC is coordinated to the boron atom.
The structure of the products was confirmed using X-ray crystallography. The team found that the phosphabora[3]dendralenes can undergo cycloadditions, e.g., with acetylene derivatives. This could be useful in the preparation of main-group-doped polycyclic aromatic hydrocarbon species.
- Heavy Heterodendralenes: Structure and Reactivity of Phosphabora[3]dendralenes,
Vesela G. Zarkina, Gary S. Nichol, Michael J. Cowley,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.4c07850