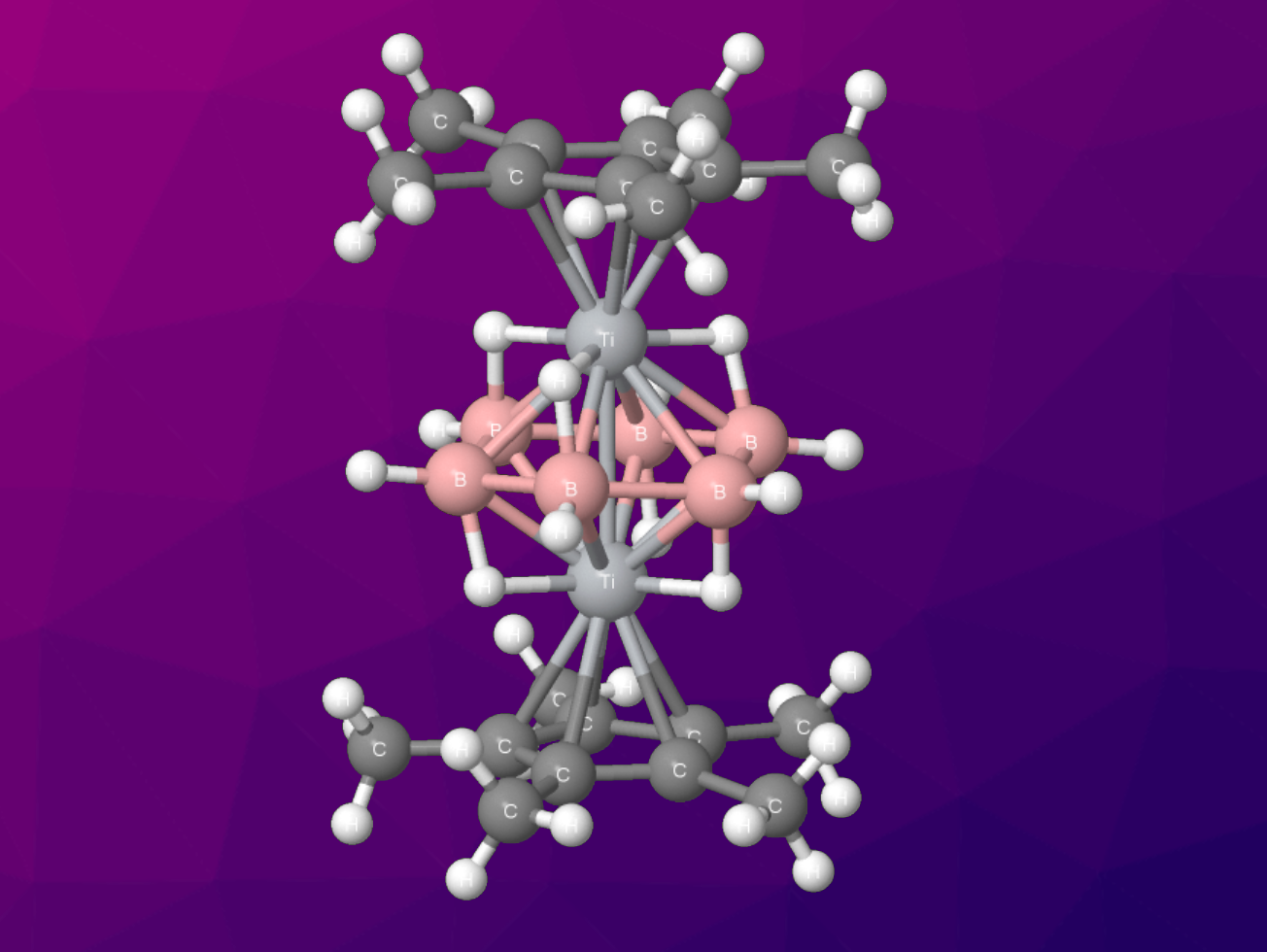
Three-dimensional polyhedral boranes dominate borane chemistry. The isolation of planar [B6H6] is a long-sought goal in boron chemistry. Several attempts in the past to stabilize [B6H6] have been unsuccessful due to the dominance of polyhedral geometries.
Sundargopal Ghosh, Indian Institute of Technology, Chennai, India, and colleagues have synthesized the first flat B6H6 unit stabilized as a part of a [(Cp*Ti)2(µ-η6:η6-B6H6)(µ-H)6] complex. To synthesize the triple-decker sandwich complex, the team treated [Cp*TiCl3] with three equivalents of [LiBH4⋅THF] at –78 °C, followed by thermolysis in the presence of excess [BH3⋅THF].
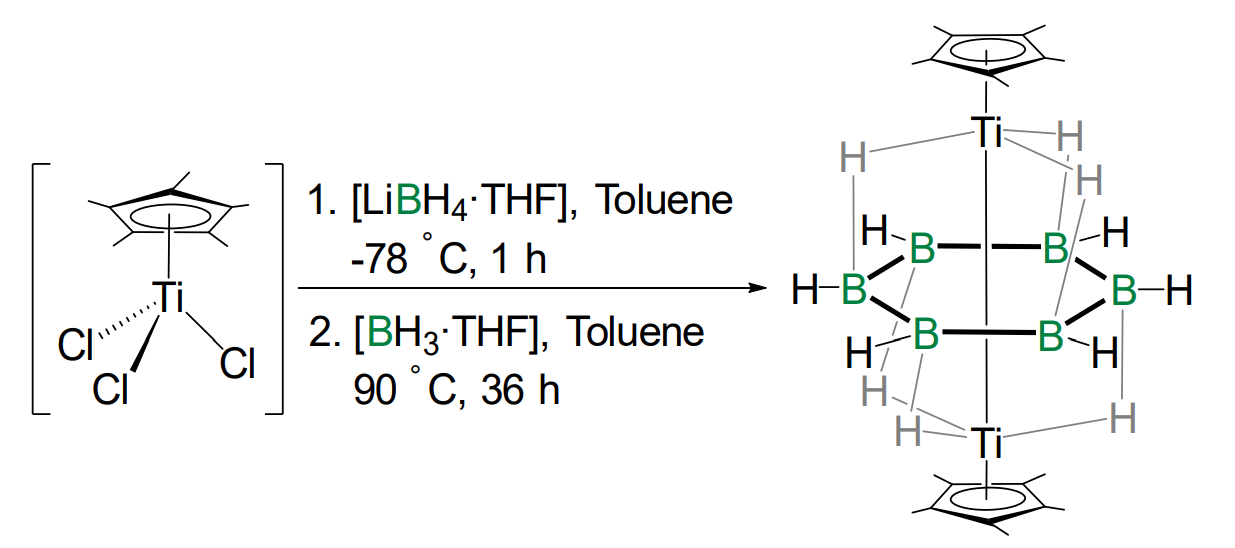
A combination of NMR spectroscopy, X-ray structure analysis, and DFT calculations revealed a B6 ring stabilized by six bridging hydrogen atoms. Significant electron delocalization was observed from the metal–metal bonding orbital to the bridging hydrogen atoms and boron–boron skeleton.
The researchers believe this research will have implications for metallaborane chemistry because it reveals that a [B6H12]6– borate anion can be isolated when it is stabilized by bridging hydrogen atoms and early transition metals such as titanium.
- Hexagonal Planar [B6H6] within a [B6H12] Borate complex; Structure and Bonding of [(Cp*Ti)2(µ‐ɳ6:ɳ6‐B6H6)(µ‐H)6],
Sourav Kar, Subhash Bairagi, Anagha Haridas, Gaurav Joshi, Eluvathingal D. Jemmis, Sundargopal Ghosh,
Angew. Chem. Int. Ed. 2022.
https://doi.org/10.1002/anie.202208293