Both carboxylate ions (RCOO–) and acetylides (RC≡C–) can serve as ligands for metal centers. So far, there had been no examples of compounds in which these two different coordination modes are realized within a single ligand. The doubly deprotonated form of propiolic acid (–C≡C-COO–) could serve as such a ligand, and is known as an intermediate in organic transformations. However, it has never been isolated and characterized in detail.
Uwe Ruschewitz, University of Cologne, Germany, and colleagues have synthesized the first crystalline compound with this elusive –C≡C-COO– anion, i.e., Na2C3O2. The team first synthesized the anhydrous sodium salt of propiolic acid, Na(OOC–C≡CH), via a reaction of propiolic acid with NaOH in methanol. This salt was then reacted with either sodium electride or NaC2H in liquid ammonia to obtain crystalline powders of Na2C3O2. The reaction with sodium electride led to a higher crystallinity of the product than the reaction with NaC2H, but also to the formation of byproducts, presumably due to the decomposition and polymerization of Na2C3O2.
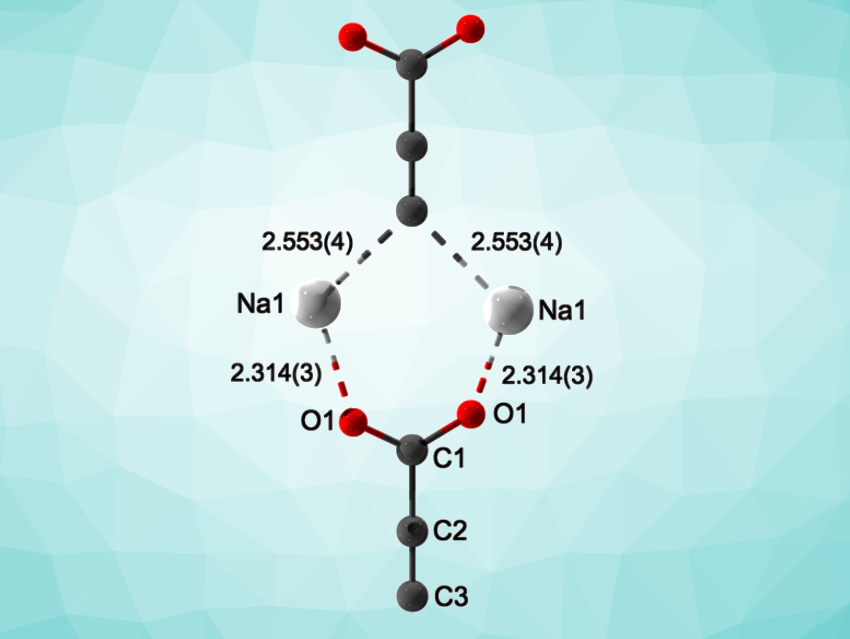
The researchers used synchrotron powder diffraction to elucidate the crystal structure of Na2C3O2. The results confirmed the formation of the Y-shaped –C≡C-COO– anion (pictured), which was previously unprecedented in crystalline materials. The product is very sensitive to air, moisture, and mechanical stress, which made further investigations difficult.
- Na2C3O2: the first crystalline compound with the elusive –C≡C‐COO– anion,
Markus Krüger, Alexandra Lamann-Glees, Sean S. Sebastian, Renée Siegel, Jürgen Senker, Jan Hempelmann, Richard Dronskowski, Uwe Ruschewitz,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202402978