Neptunium is a synthetic, radioactive element and an actinide metal. Its coordination chemistry is not well developed in all accessible oxidation states. High-valent neptunium generally requires stabilization from at least one metal–ligand multiple bond.
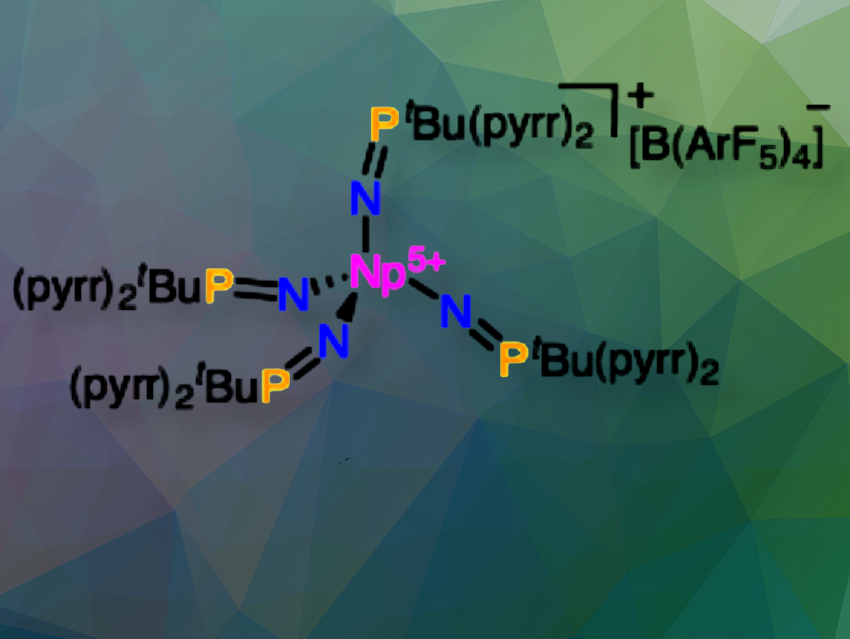
Ivan A. Popov, The University of Akron, OH, USA, Henry S. La Pierre, Georgia Institute of Technology, Atlanta, GA, USA, and Pacific Northwest National Laboratory, Richland, WA, USA, and colleagues have synthesized a tetrahedral molecular neptunium(V) complex (pictured) ([Np5+(NPC)4][B(ArF5)4], 1-Np, with NPC = [NPtBu(pyrr)2]−; tBu = C(CH3)3; pyrr = pyrrolidinyl (N(C2H4)2); B(ArF5)4 = tetrakis(2,3,4,5,6-pentafluourophenyl)borate).
The researchers combined Np(NPC)₄ and ferrocenium tetrakis(pentafluorophenyl)borate ([Cp2Fe][B(ArF5)4]) as a fairly mild oxidant in tetrahydrofuran inside a negative pressure glovebox to receive 1-Np. It is stable in the solid state and undergoes a relatively slow, first-order proton-coupled electron transfer (PCET) reaction when stirred for 42 hours in tetrahydrofuran (THF) to form 2-Np, [Np(IV)(NPC)3(HNPC)][B(ArF5)4].
The researchers used single-crystal X-ray diffraction, solution-state spectroscopy, and density functional theory to study the neptunium(V) complex 1-Np and its reaction product 2-Np. These techniques revealed how the unique bonding in these compounds stabilizes the reactive ion and provided detailed information on the energy changes and reaction rates involved in the PCET process.
1-Np crystallizes in the P21/n space group and is isotypic with the structure [U5+(NPC)4][B(ArF5)4]. The asymmetric unit contains one [Np5+(NPC)4]+ cation and one [B(ArF5)4]− anion. 2-Np is tetrahedral.
- A tetrahedral neptunium(V) complex,
Julie E. Niklas, Kaitlyn S. Otte, Chad M. Studvick, Sabyasachi Roy Chowdhury, Bess Vlaisavljevich, John Bacsa, Florian Kleemiss, Ivan A. Popov, Henry S. La Pierre,
Nature Chem. 2024.
https://doi.org/10.1038/s41557-024-01529-6




That’s fascinating research! The synthesis of the tetrahedral molecular neptunium(V) complex is a significant contribution to the field of
coordination chemistry. It’s particularly interesting to see how the unique bonding in these compounds stabilizes the reactive ion, as revealed through single-crystal X-ray diffraction, solution-state spectroscopy,
and density functional theory. The detailed information on the energy changes and reaction rates involved in the proton-coupled electron transfer process
is also quite insightful. I’m looking forward to seeing the future applications of this
research.