Polycyclic aromatic hydrocarbons (PAHs) can have applications, e.g., in organic electronics. To tune their electronic properties, PAHs can, for example, be doped with nitrogen atoms. In this context, coronene derivatives are interesting candidates as chromophores. However, nitro-atom doping of coronene derivatives has been rare.
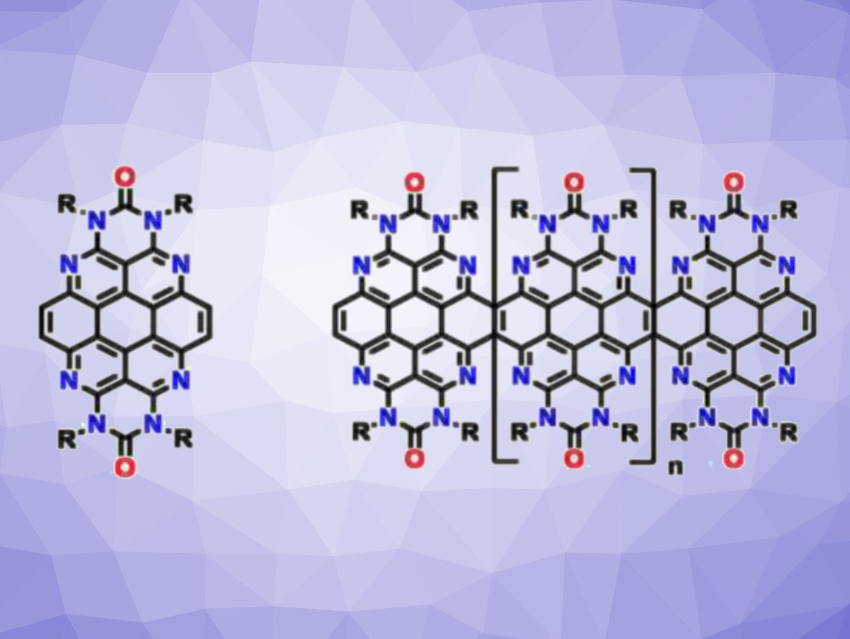
Lutz H. Gade, University of Heidelberg, Germany, and colleagues have synthesized tetraazacoronenes as well as a condensed azacoronene dimer and higher oligomers (examples pictured). The team prepared the desired tetraazacoronenes via two different strategies. First, cyclobutene-annulated azacoronenes were obtained from bay-functionalized tetraazaperylenes via a zirconium-mediated cyclization. The researchers started from an octaazaperopyrenedioxide (OAPPDO) derivative. which was alkynylated via a coupling with trimethyl((tributylstannyl)ethynyl)silane and then underwent a zirconium-mediated cyclization using Cp2ZrCl2 and n-butyllithium.
In a second approach, the team used a four-fold Suzuki-Miyaura cross-coupling of the OAPPDO derivative and (E)-(1,2-bis(pinacolatoboryl)vinyl)trimethylsilane, followed by desilylation. This reaction gave the target azacoronene in a yield of 35 %, the corresponding dimer in a yield of 36 %, as well as small amounts of the trimer and tetramer. The products showed pronounced fluorescence properties. They might be a useful starting point for further expansion towards nanoribbons with adjustable optoelectronic properties.
- Tetraazacoronenes and Their Dimers, Trimers and Tetramers,
Robert Eichelmann, Joachim Ballmann, Lutz H. Gade,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202309198


![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)
