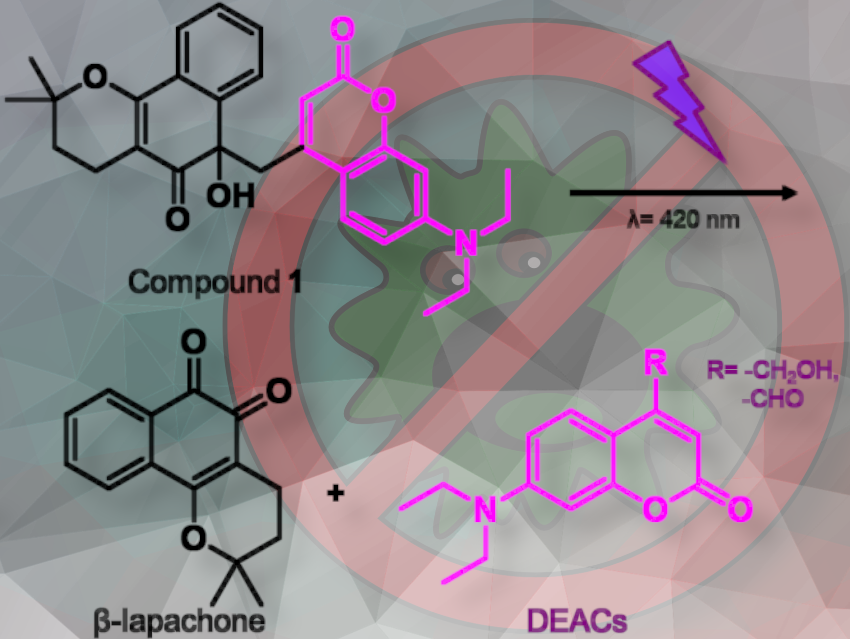
Biofilms are communities of microorganisms and molecules protected by a self-produced matrix. Their complexity makes them resistant to conventional antimicrobial treatments. Recently, the natural product β-lapachone was identified as a biofilm-eradicating agent by inhibiting the catalase enzyme. However, β-lapachone has downsides, such as off-target toxicity in vivo. Using a prodrug approach, these problems could be avoided. A prodrug is a pharmacologically inactive compound that is metabolized in the body to produce an active drug.
Andrew A Beharry, University of Toronto, Canada, and colleagues previously the use of the photolabile protecting group diethylaminocoumarin (DEAC) on β-lapachone to render its biological activity inert. This photocaged β-lapachone renders β-lapachone biologically inactive until it is exposed to a specific wavelength of light. The light exposure breaks the photolabile bond, releasing the active β-lapachone molecule.
They report that 420 nm irradiation (violet light) uncages photocaged β-lapachone, leading to Bacillus subtilis biofilm inhibition at a similar effective concentration to that of native β-lapachone. Notably, in the dark, the photocage β-lapachone does not inhibit catalase, and thus no biofilm inhibition is observed.
Overall, photocaged β-lapachone is a promising, light-activated antibacterial agent that could help fight infections related to biofilms on medical devices and wounds.
- Antimicrobial Efficacy of Photocaged β-lapachone in Bacillus subtilis Biofilms,
Elyse Hudson, Christabel Faylinn, Ivonne Rebeca Lopez-Miranda, Josh Milstein, Andrew Beharry,
ChemPhotoChem 2024.
https://doi.org/10.1002/cptc.202400164