Zeolites are commonly used solid catalysts. Their pore sizes correspond to the sizes of different molecules, allowing control over reaction selectivity. However, the confined space can limit the transport of reactants and products. The synthesis of nanozeolites with just a few unit cells is a promising strategy to solve this problem. There are examples of nanozeolites with sizes of 4–8 nm, about two to four unit cells. This poses the question: What is the smallest zeolite that could be obtained by hydrothermal synthesis?
Valeria Molinero and colleagues, University of Utah, Salt Lake City, USA, have addressed this question using thermodynamic theory and molecular dynamics (MD) simulations. The simulations predict that amorphous precursors as small as 4 nm can crystallize into zeolites, which is in agreement with experiments.
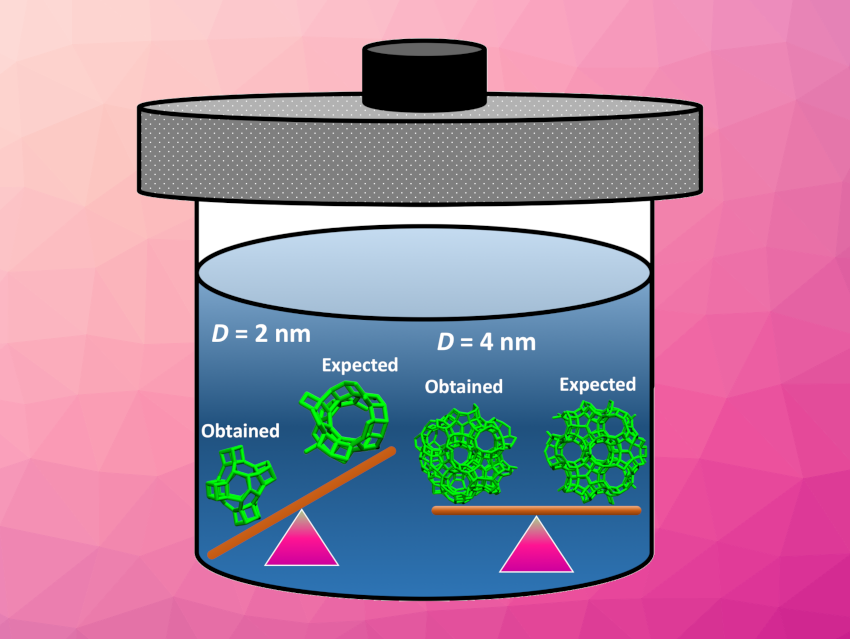
In contrast, zeolites smaller than approximately 3 ± 0.5 nm are outcompeted in stability by more compact isomers (pictured above on the left). At this size, interfacial forces dominate, leading to structural reorganization. These compact isomers have different ring and pore distributions than open-framework zeolites, and thus, are not expected to provide similar catalytic selectivity and efficiency.
In summary, according to the researchers, zeolites smaller than 3 ± 0.5 nm cannot be obtained through hydrothermal synthesis. This conclusion should apply to the synthesis of a broad range of ultra-small zeolites.
- What is the Smallest Zeolite that Could be Synthesized?,
Debdas Dhabal, Andressa A. Bertolazzo, Valeria Molinero,
Angew. Chem. Int. Ed. 2022.
https://doi.org/10.1002/anie.202205095