α-Nitroketones have a strong electron-withdrawing group adjacent to the carbonyl group, enabling them to act as good nucleophiles in several important reactions, such as Michael reactions, inverse electron-demanding Diels–Alder reactions, and Mannich reactions. They are also useful precursors in the synthesis of, e.g., β-nitroalcohols and α-aminoketones. However, the preparation of α-nitroketones usually requires harsh, environmentally harmful conditions, making new synthetic approaches interesting research targets.
Yujiro Hayashi, Tohoku University, Sendai, Japan, and colleagues have developed an environmentally friendly synthesis of α-nitroketones using simple reagents. The team used α-substituted malononitriles, which were oxidized in the presence of nitromethane, potassium tert-butoxide, and molecular oxygen to form an acyl cyanide intermediate. This intermediate undergoes nucleophilic acyl substitution with nitronate, which is generated in situ by the deprotonation of nitromethane.
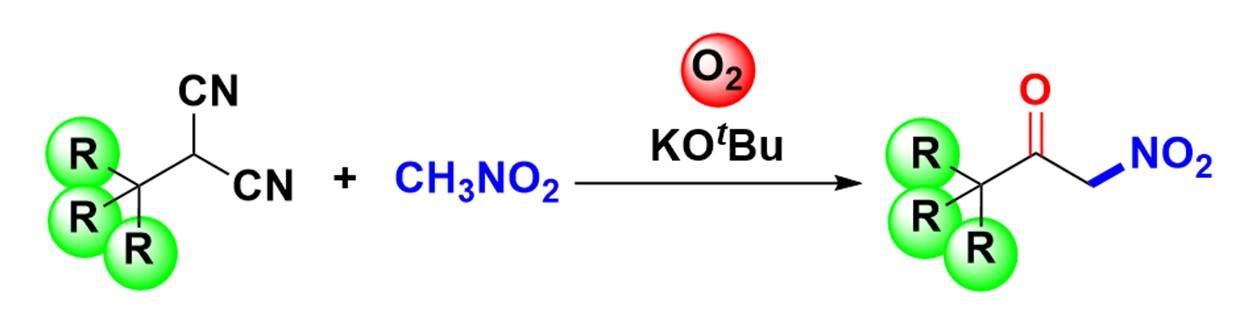
The desired products were obtained in good yields. The approach also enables the otherwise challenging synthesis of sterically hindered α-nitroketones with quaternary carbons at the α’-position.
- Oxidative Synthesis of α‐Nitroketones from α‐Substituted Malononitrile and Nitromethane Using Molecular Oxygen without Condensation Reagents,
Yujiro Hayashi, Emanuele Cocco, Hinata Odaira, Hiroaki Matoba, Naoki Mori,
European Journal of Organic Chemistry 2023.
https://doi.org/10.1002/ejoc.202300964



![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)