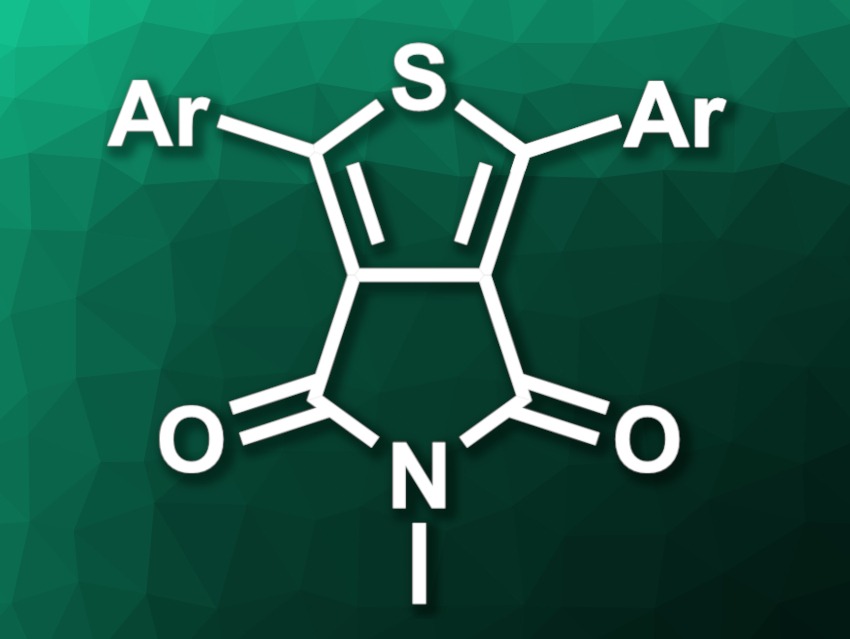
Thieno[3,4-c]pyrrole-4,6-dione (TPD) derivatives contain a thiophene ring and a dione-functionalized pyrrolidine ring fused to one another. These compounds can be useful building blocks for donor–acceptor copolymers. Existing methods for the synthesis of TPD derivatives have drawbacks such as a need for multiple steps, toxic reagents, or transition-metal catalysts.
Fuhong Xiao, Guo-Jun Deng, Xiangtan University, China, and colleagues have developed a three-starting-material four-component reaction for the construction of thienopyrrolediones from aromatic aldehydes, 1-methylpyrrolidin-2-one (NMP), and elemental sulfur under transition-metal-free conditions. The team reacted a variety of substituted aryl aldehydes with S8 and NMP, which was also used as the solvent, in the presence of iodine and di-tert-butyl peroxide (CTBP). Cs2CO3 was used as a base and water as an additive. The reactions were performed at 150 °C.
The desired TPD derivatives (general structure pictured) were obtained in moderate to good yields, depending on the aldehyde used. The team proposed a reaction mechanism in which NMP is first oxidized to N-methyl succinimide, which then reacts sequentially with two equivalents of the aldehyde. The resulting intermediate reacts with the sulfur in a [4 + 1]-type annulation reaction to give the product. According to the researchers, the approach could provide a relatively green and efficient path to TPD-containing functional materials.
- A Four-Component Reaction for the Synthesis of Thienopyrrolediones under Transition Metal Free Conditions,
Qiaoyan Xing, Fuhong Xiao, Guojiang Mao, Guo-Jun Deng,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.2c01599



![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)