Natural products containing a naphthalene core are relatively rare. However, many of these natural-occurring molecules show various kinds of pharmacological activities, and thus, attract the attention of synthetic chemists. Existing synthetic routes generally start from substituted benzene derivatives and require extra steps to construct the naphthalene scaffold via aromatization reactions. Suitable methods for the synthesis of such compounds directly from available naphthalene derivatives could save labor and lower costs.
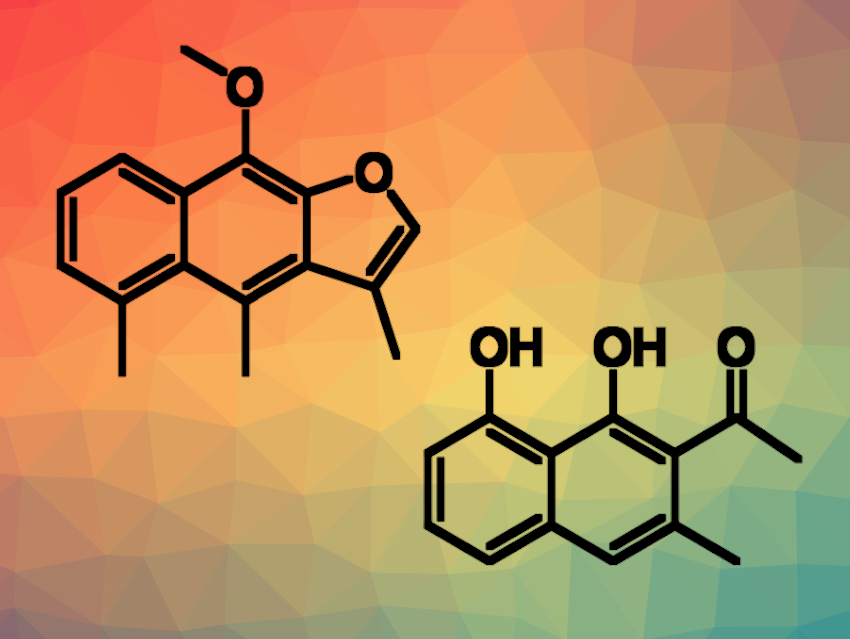
Yinan Zhang, Nanjing University of Chinese Medicine, Jiangsu, China, and colleagues have developed an efficient approach to the methylated naphthalene natural products dehydrocacalohastine and musizin (pictured; dehydrocacalohastine on the left, musizin on the right). The desired products were obtained from simple, commercially available naphthalene derivatives, i.e., 1-bromonaphthalen-2-ol and naphthalene-1,8-diol. In a key step of both syntheses, the researchers employed a regioselective peri– or ortho-C−H methylation of 1-naphthaldehydes using a transient ligand strategy.
Overall, the team obtained dehydrocacalohastine in 8 steps and 8.9 % overall yield and musizin in 8 steps and yields up to 27 %. The synthetic route tolerates various substituents on the naphthalene ring. According to the researchers, this could provide an opportunity to readily prepare natural analogues for in-depth medicinal studies of these useful scaffolds.
- Synthesis of naphthalene natural products dehydrocacalohastine and musizin,
Yujian Mao, Zhen Xia, Lihong Hu, Yinan Zhang,
Asian J. Org. Chem. 2022.
https://doi.org/10.1002/ajoc.202200198



![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)