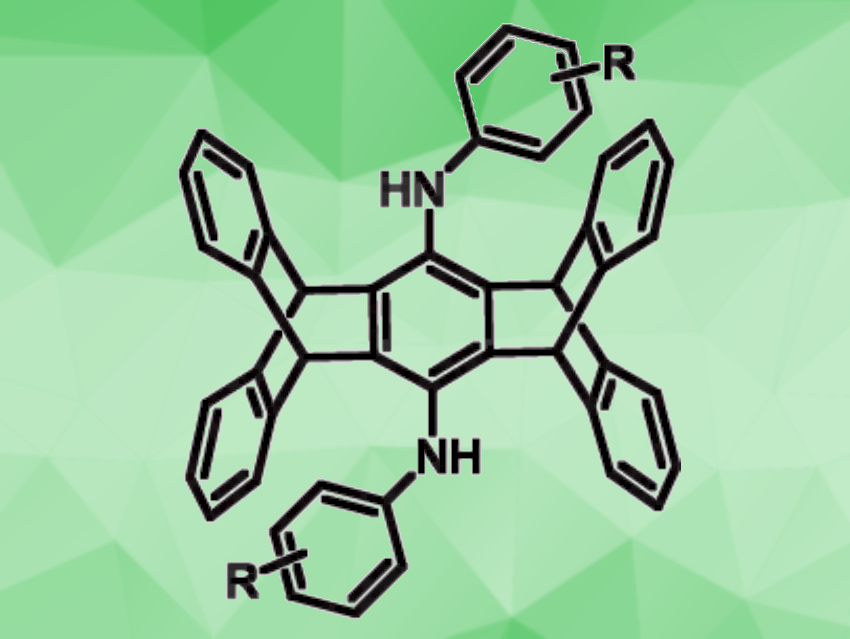
Iptycenes are aromatic compounds that consist of arene units bound to a bicyclooctatriene core. Pentiptycene, for example, features two of those cores connected via one benzene ring and contains four more aromatic rings around the periphery. Pentiptycenes can be useful in supramolecular chemistry and in functional materials. Pentiptycene quinone can be readily prepared as a precursor for the preparation of different pentiptycenes, but these synthetic pathways can require multiple steps and give relatively low yields.
Jye-Shane Yang, National Taiwan University, Taipei, and colleagues have developed a method for the synthesis of bis(arylamino)pentiptycenes (general structure pictured) from pentiptycene quinone that can be achieved in a single step. The team used a TiCl4-DABCO-assisted reductive amination with anilines (DABCO = 1,4-diazabicyclo[2.2. 2]octane) to prepare the desired products. They reacted pentiptycene quinone with different aniline derivatives in the presence of six equivalents each of TiCl4 and DABCO, using PhCl as the solvent. The reactions were performed at 140 °C.
The desired bisfunctionalized pentiptycenes were obtained in moderate to very high yields. These products could be further transformed, for example, for the development of organic electronic materials. According to the researchers, the transformation involves a dual amination of pentiptycene quinone to give quinone diimines, followed by an in-situ reduction of the quinone diimines—both unprecendented.
- One-Pot Synthesis of Bis(arylamino)pentiptycenes by TiCl4-DABCO Assisted Reductive Amination of Pentiptycene Quinone,
Zhe-Jie Zhang, Ying-Feng Hsu, Chia-Chien Kao, Jye-Shane Yang,
Org. Lett. 2024.
https://doi.org/10.1021/acs.orglett.4c00939